DIETHYLALUMINUM CHLORIDE
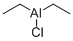
- CAS No.
- 96-10-6
- Chemical Name:
- DIETHYLALUMINUM CHLORIDE
- Synonyms
- DEAC;DIETHYLALUMINIUM CHLORIDE;Diethylaluminum;uminum chL;Diethylchoroaluminum;CHLORODIETHYLALUMINUM;chlorodiethyl-aluminu;Diethylchloroaluminum;Et2AlCl 1M in heptane;chloro(diethyl)alumane
- CBNumber:
- CB9783672
- Molecular Formula:
- C4H10AlCl
- Molecular Weight:
- 120.56
- MOL File:
- 96-10-6.mol
- MSDS File:
- SDS
- Modify Date:
- 2023/10/21 15:54:43
| Melting point | -85°C |
|---|---|
| Boiling point | 125°C 50mm |
| Density | 0.887 g/mL at 25 °C |
| vapor pressure | 3 mmHg ( 60 °C) |
| Flash point | −9 °F |
| storage temp. | 0-6°C |
| solubility | Miscible with hexane. |
| form | Solution |
| Specific Gravity | 0.711 (20/4℃) |
| color | Colorless |
| Water Solubility | reac H2O [CRC10] |
| Sensitive | Air & Moisture Sensitive |
| Merck | 326 |
| BRN | 4123259 |
| CAS DataBase Reference | 96-10-6(CAS DataBase Reference) |
| EPA Substance Registry System | Aluminum, chlorodiethyl- (96-10-6) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |     GHS02,GHS05,GHS07,GHS08 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H225-H250-H260-H304-H314-H336-H361d-H373-H412 | |||||||||
| Precautionary statements | P210-P231+P232-P280-P301+P330+P331-P303+P361+P353-P304+P340+P310-P305+P351+P338-P370+P378 | |||||||||
| Hazard Codes | F,C,N | |||||||||
| Risk Statements | 14-17-34-67-65-62-51/53-48/20-14/15-11-63-50/53 | |||||||||
| Safety Statements | 36/37/39-45-62-61-43-26-16 | |||||||||
| RIDADR | UN 3394 4.2/PG 1 | |||||||||
| WGK Germany | 2 | |||||||||
| RTECS | BD0558000 | |||||||||
| F | 10 | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 4.3 | |||||||||
| PackingGroup | I | |||||||||
| HS Code | 29319090 | |||||||||
| NFPA 704 |
|
DIETHYLALUMINUM CHLORIDE Chemical Properties,Uses,Production
Chemical Properties
colorless solution
Production Methods
Ethylaluminum sesquichloride (26.5 kg) added to a nitrogen-purged reactor, was heated to 175 ℃. Then, while the mixture was stirred vigorously, 1.1 kg sodium was added over a 30 min period; the mixture was further heated at 155 – 190 ℃ for 60 min. The diethylaluminum chloride product distilled from the reactor at 100 – 161 ℃ (1.3 – 6.1 kPa). In this example, an excess of ethylaluminum sesquichloride was employed to facilitate draining the voluminous byproduct salt and aluminum solids from the reactor. In an alternate approach, a heavy hydrocarbon oil, added prior to reaction, may be employed to remove the solids in slurry form, permitting the use of a stoichiometric ratio of ethylaluminum sesquichloride and sodium reactants.
General Description
Colorless liquid. Dangerous fire and explosion hazard. Used as an intermediate in production of organometallics.
Air & Water Reactions
Pyrophoric in air [Hawley]. Reacts violently with water, Rose(1961).
Reactivity Profile
Organometallics, such as DIETHYLALUMINUM CHLORIDE, are reactive with many other groups. Incompatible with acids and bases. Organometallics are good reducing agents and therefore incompatible with oxidizing agents. Often reactive with water to generate toxic or flammable gases. Organometallics containing halogens (fluorine, chlorine, bromine, iodine) bonded to the metal typically with generate gaseous hydrohalic acids (HF, HCl, HBr, HI) with water.
Potential Exposure
These materials are used as components of olefin polymerization catalysts. The reader is referred to the entry on “Aluminum alkyls” for additional information on this entry. The aluminum alkyl halides parallel very closely the aluminum alkyls
Shipping
UN3052 Spontaneously combustible. Water reactive releasing large quantities of toxic and deadly hydrogen gas. (Note: this number does not appear in the 49/CFR HazMat tables)
Purification Methods
Distil it from excess dry NaCl (to remove ethyl aluminium dichloride) in a 50-cm column containing a heated nichrome spiral. [Beilstein 4 IV 4403.]
Incompatibilities
The aluminum alkyl halides are strong reducing agents; they react—possibly violently—with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides. These chemicals react violently with nitromethaneEthylaluminum sesquichloride reacts explosively with carbon tetrachloride at room temperature. This chemical reacts violently with water, forming corrosive hydrogen chloride and flammable ethane gas. Diethylaluminum chloride may form an explosive product with chlorine azide.
DIETHYLALUMINUM CHLORIDE Preparation Products And Raw materials
Raw materials
1of2
chevron_rightPreparation Products
1of2
chevron_right| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| HYCHEM SAINOR CHEMICALS PVT LTD | +919391007257 | Telangana, India | 205 | 58 | Inquiry |
| CLEARSYNTH LABS LTD. | +91-22-45045900 | Hyderabad, India | 6351 | 58 | Inquiry |
| Alfa Aesar | 1 800 209 7001 | Maharashtra, India | 6913 | 58 | Inquiry |
| Sainor Laboratories Private Limited | 09666644167 | Hyderabad, India | 66 | 58 | Inquiry |
| VASIN BIO LIFE SCIENCES PVT LTD | 9491644688 | Hyderabad, India | 2096 | 58 | Inquiry |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | China | 21661 | 55 | Inquiry |
| career henan chemical co | +86-0371-86658258 +8613203830695 | China | 29899 | 58 | Inquiry |
| Chongqing Chemdad Co., Ltd | +86-023-6139-8061 +86-86-13650506873 | China | 39916 | 58 | Inquiry |
| SIMAGCHEM CORP | +86-13806087780 | China | 17367 | 58 | Inquiry |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418671 +8618949823763 | China | 34571 | 58 | Inquiry |
96-10-6(DIETHYLALUMINUM CHLORIDE)Related Search:
1of4
chevron_right




