ETHYLALUMINUM SESQUICHLORIDE
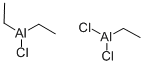
- CAS No.
- 12075-68-2
- Chemical Name:
- ETHYLALUMINUM SESQUICHLORIDE
- Synonyms
- EASC;5%soln.inheptane;uminum sesquichL;dichloro(ethyl)alumane;Trichlorotriethyldialuminum;trichlorotriethyldi-aluminu;sesquiethylaluminumchloride;Triethyltrichlorodialuminum;Aluminiumethylsesquichlorid;Sesquiethylaluminum chloride
- CBNumber:
- CB1135646
- Molecular Formula:
- C6H15Al2Cl3
- Molecular Weight:
- 247.51
- MOL File:
- 12075-68-2.mol
- MSDS File:
- SDS
- Modify Date:
- 2023/10/21 15:50:51
| Melting point | −50 °C(lit.) |
|---|---|
| Boiling point | 204 °C(lit.) |
| Density | 0.904 g/mL at 25 °C |
| Flash point | −1 °F |
| storage temp. | water-free area |
| form | liquid |
| color | Yellow liquid |
| BRN | 4027369 |
| CAS DataBase Reference | 12075-68-2 |
| EPA Substance Registry System | Aluminum, di-.mu.-chlorochlorotriethyldi- (12075-68-2) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS02,GHS05 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H250-H260-H314 | |||||||||
| Precautionary statements | P210-P231+P232-P280-P303+P361+P353-P305+P351+P338-P370+P378 | |||||||||
| Hazard Codes | F,C | |||||||||
| Risk Statements | 15-17-23/24/25-34-14/15-14-67-65-48/20-11-63-62-51/53-35 | |||||||||
| Safety Statements | 16-26-36/37/39-45-27-62-43-57-43E-29-6A | |||||||||
| RIDADR | UN 3394 4.2/PG 1 | |||||||||
| WGK Germany | 1 | |||||||||
| RTECS | BD1950000 | |||||||||
| HazardClass | 4.2 | |||||||||
| PackingGroup | I | |||||||||
| HS Code | 29319090 | |||||||||
| NFPA 704 |
|
ETHYLALUMINUM SESQUICHLORIDE Chemical Properties,Uses,Production
Chemical Properties
clear colorless solution
Uses
Catalyst for olefin polymerization, aromatic hydrogenation; intermediate.
Production Methods
Ethyl chloride and aluminum could used as starting metarials to synthesize Ethylaluminum sesquichloride. A 9.5 × 10-2 m3 closed steel reactor, fitted with a reflux condenser and equipped for vacuum distillation of product, was first purged with nitrogen. After addition of 13.6 kg of aluminum powder and a catalyst mixture composed of 3.6 kg diethylaluminum chloride and 410 g iodine, the reactor contents were stirred and heated slowly to 130 ℃. The temperature was maintained at 120 – 150 ℃ during addition of 50 kg ethyl chloride over a period of 3 h. The product can be flash distilled under vacuum, or it can be drained from the reactor and clarified by settling or by filtration of black residual metal solids. Iodine can be omitted from the procedure, in which case the induction period may be increased.
General Description
A clear yellow liquid. Slightly denser than water. Used to make other chemicals.
Air & Water Reactions
Spontaneously flammable in air (Douda 1966). Reacts violently with water forming hydrogen chloride and flammable ethane gas (Rose 1961).
Reactivity Profile
Organometallics, such as ETHYLALUMINUM SESQUICHLORIDE, are reactive with many other groups. Incompatible with acids and bases. Organometallics are good reducing agents and therefore incompatible with oxidizing agents. Often reactive with water to generate toxic or flammable gases. Organometallics containing halogens (fluorine, chlorine, bromine, iodine) bonded to the metal typically with generate gaseous hydrohalic acids (HF, HCl, HBr, HI) with water. A mixture of ETHYLALUMINUM SESQUICHLORIDE with carbon tetrachloride exploded when warmed to room temperature [Bretherick, 5th Ed., 1995].
Hazard
Ignites on contact with air, dangerous fire risk, reacts violently with water.
Health Hazard
Inhalation of smoke from fire causes metal-fume fever (flu-like symptoms); acid fumes irritate nose and throat. Contact with liquid, which is spontaneously flammable, causes severe burns of eyes and skin.
Safety Profile
Mixtures with carbon tetrachloride explode at room temperature. When heated to decomposition it emits toxic fumes of Cl-. See also ALUMINUM COMPOUNDS.
Potential Exposure
These materials are used as components of olefin polymerization catalysts. The reader is referred to the entry on “Aluminum alkyls” for additional information on this entry. The aluminum alkyl halides parallel very closely the aluminum alkyls
Shipping
UN3052 Spontaneously combustible. Water reactive releasing large quantities of toxic and deadly hydrogen gas. (Note: this number does not appear in the 49/CFR HazMat tables)
Incompatibilities
The aluminum alkyl halides are strong reducing agents; they react—possibly violently—with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides. These chemicals react violently with nitromethaneEthylaluminum sesquichloride reacts explosively with carbon tetrachloride at room temperature. This chemical reacts violently with water, forming corrosive hydrogen chloride and flammable ethane gas. Diethylaluminum chloride may form an explosive product with chlorine azide.
ETHYLALUMINUM SESQUICHLORIDE Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Alfa Aesar | 1 800 209 7001 | Maharashtra, India | 6913 | 58 | Inquiry |
| Shanxi Naipu Import and Export Co.,Ltd | +86-13734021967 +8613734021967 | China | 1011 | 58 | Inquiry |
| career henan chemical co | +86-0371-86658258 +8613203830695 | China | 29826 | 58 | Inquiry |
| Hefei TNJ Chemical Industry Co.,Ltd. | 0551-65418671 | China | 34571 | 58 | Inquiry |
| ANHUI WITOP BIOTECH CO., LTD | +8615255079626 | China | 23556 | 58 | Inquiry |
| Shaanxi Dideu Medichem Co. Ltd | +86-029-89586680 +86-18192503167 | China | 9030 | 58 | Inquiry |
| Hebei Guanlang Biotechnology Co., Ltd. | +86-19930503259 +86-19930503259 | China | 18446 | 58 | Inquiry |
| Aladdin Scientific | +1-+1(833)-552-7181 | United States | 57511 | 58 | Inquiry |
| Aladdin Scientific | +1-+1(833)-552-7181 | United States | 52927 | 58 | Inquiry |
| Hebei Fengmu Trading Co., Ltd. | +8613393234347 | China | 2989 | 58 | Inquiry |





