Triethylaluminum
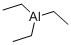
- CAS No.
- 97-93-8
- Chemical Name:
- Triethylaluminum
- Synonyms
- TRIETHYLALUMINIUM;Triethylaluminum,95%,LIQUID;uminum;triethylalane;triethyl-alane;Triethylalumine;triethylalumane;TRIETHYLALUMINUM;triethyl-aluminu;aluminiumtriethyl
- CBNumber:
- CB3854738
- Molecular Formula:
- C6H15Al
- Molecular Weight:
- 114.16
- MOL File:
- 97-93-8.mol
- Modify Date:
- 2023/11/28 16:31:44
| Melting point | -50°C |
|---|---|
| Boiling point | 128-130°C (50 mmHg) |
| Density | 0.85 g/mL at 20 °C |
| vapor pressure | 1 mmHg ( 62.2 °C) |
| Flash point | −1 °F |
| storage temp. | 0-6°C |
| form | liquid |
| color | colorless |
| Water Solubility | reacts |
| Sensitive | Air & Moisture Sensitive |
| BRN | 3587229 |
| Dielectric constant | 6.4(20℃) |
| Exposure limits |
ACGIH: TWA 50 ppm (Skin) OSHA: TWA 500 ppm(1800 mg/m3) NIOSH: IDLH 1100 ppm; TWA 50 ppm(180 mg/m3) |
| InChIKey | VOITXYVAKOUIBA-UHFFFAOYSA-N |
| CAS DataBase Reference | 97-93-8(CAS DataBase Reference) |
| NIST Chemistry Reference | Triethylaluminum(97-93-8) |
| EPA Substance Registry System | Triethylaluminum (97-93-8) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |     GHS02,GHS05,GHS07,GHS08 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H225-H250-H252-H260-H304-H314-H336-H361d-H373-H412 | |||||||||
| Precautionary statements | P210-P231+P232-P280-P301+P330+P331-P303+P361+P353-P304+P340+P310-P305+P351+P338-P370+P378 | |||||||||
| Hazard Codes | F,C,N | |||||||||
| Risk Statements | 14-17-20/21/22-34-67-65-51/53-11-63-48/20-62-14/15-23/24/25-50/53-10 | |||||||||
| Safety Statements | 26-45-62-6A-43A-36/37/39-24/25-16-43-61-33-46 | |||||||||
| RIDADR | UN 3394 4.2/PG 1 | |||||||||
| WGK Germany | 2 | |||||||||
| RTECS | BD2050000 | |||||||||
| F | 10-21 | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 4.3 | |||||||||
| PackingGroup | I | |||||||||
| HS Code | 29319090 | |||||||||
| Toxicity | LC50 ihl-rat: 10 g/m3/15M 85JCAE -,1216,86 | |||||||||
| NFPA 704 |
|
Triethylaluminum Chemical Properties,Uses,Production
Chemical Properties
Colorless liquid. Miscible with saturated hydrocarbons.
Uses
Triethylaluminum, in combination withmany transition metal complexes, is used as Ziegler-Natta polymerization and hydrogenationcatalyst. Also, it is used as intermediatein organic syntheses.
Production Methods
In a typical example, a slurry is prepared containing 7 wt % aluminum powder (0.1 wt% zirconium catalyst content) and 45 wt% recycled triethylaluminum in a paraffin hydrocarbon fraction, boiling range 177 – 260 ℃. The slurry is pumped into the reactor at a rate of 1.5 m3/h, at a reactor temperature of 132 ℃, while hydrogen is introduced at the bottom to maintain a partial pressure of 10 MPa. Excess hydrogen is removed in flash drum, wherein pressure is decreased to 3 MPa and temperature is lowered to prevent thermal reversal of the reaction. In the ethylation reactor sufficient ethylene is added to react completely with the Al – H bonds. Temperature is held at around 85 ℃ or somewhat higher. The addition rate increases with temperature, but carboalumination of ethylene also can occur to formn-butyl groups, especially at higher temperatures. Hydrogen and ethylene partial pressure is kept low in ethylation reactor to minimize hydrogenation of ethylene to ethane and to reduce excess carboalumination products. Excess ethylene and hydrogen are removed in flash drum. A portion of the triethylaluminum product solution is recycled to reactor.
General Description
A colorless liquid. Flammable gas is produced on contact with water.
Air & Water Reactions
Pyrophoric, ignites in moist air [Merck 11th ed. 1989]. Reacts violently with water.
Reactivity Profile
Triethylaluminum reacts violently with water, alcohols, phenols, amines, carbon dioxide, sulfur oxides, nitrogen oxides, halogens, and halogenated hydrocarbons, causing fire and explosion hazards. [Handling Chemicals Safely 1980 p. 929]. A mixture of dimethylformamide and triethyl aluminum exploded when heated [Bretherick 1995].
Health Hazard
Exposure to smoke from fire causes metal-fume fever (flu-like symptoms). Since liquid ignites spontaneously, contact with eyes or skin causes severe burns.
Fire Hazard
Triethylaluminum is extremely pyrophoric, igniting spontaneously in air. It reacts violently with water, alcohol, halogenated hydrocarbons, and oxidizing substances. Among the alcohols, the lower alcohols, methanol, ethanol, n- propanol, and isopropyl alcohol, react explosively with triethylaluminum. Reactions with lower aldehydes, ketones and amides can be vigorous to violent. It may explode on contact with halocarbons in excess molar ratios or upon slight warming. When heated to 200°C (392°F), it decomposes, liberating ethylene and hydrogen.
Safety Profile
Extremely destructive to living tissue. A very dangerous fire hazard when exposed to heat or flame. Ignites spontaneously in air. Explodes violently in water. To fight fire, use CO2, dry sand, dry chemical. Do not use water, foam, or halogenated fire-fighting agents. Explosive reaction with alcohols (e.g., methanol, ethanol, propanol), carbon tetrachloride, N,N-dmethylformamide + heat. Incompatible with halogenated hydrocarbons; triethyl borane. When heated to decomposition it emits acrid smoke and irritating fumes. See also ALUMINUM COMPOUNDS and ORGANOMETALS.
Potential Exposure
Alkyl aluminum compounds are used as components of olefin polymerization catalysts. They are also used in the synthesis of higher primary alcohols and in pyrophoric fuels, as a catalyst in making ethylene gas; and in plating aluminum.
Shipping
ntial fire or explosion hazard. Shipping: UN3399 Organometallic substance, liquid, water-reactive, flammable, Hazard Class: 4.3; Labels: 4.3 Dangerous Dangerous when wet material, 3-Flammable liquid, technical name Required. UN3051-Spontaneously combustible. Also, this material is dangerous when wet. (Note: this number does not appear in the 49/CFR HazMat tables).
Purification Methods
Purify it by fractionation in an inert atmosphere under a vacuum in a 50cm column containing a heated nichrome spiral, taking the fraction b 112-114o/27mm. It is very sensitive to H2O and should be stored under N2. It should not contain chloride ions which can be shown by hydrolysis and testing with AgNO3. [Baker & Sisler J Am Chem Soc 75 4828 5193 1953, NMR: Brownstein et al. J Am Chem Soc 81 3826 1959, Beilstein 4 IV 4398.]
Incompatibilities
The lighter trialkylaluminums ignite spontaneously in air; can self-heat in the air at room temperature without any added energy and may ignite. These compounds are strong reducing agents. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides. Incompatible with water, oxygen (air), acids, alcohols, phenols, amines, carbon dioxide; sulfur oxides; halogenated compounds, and many other substances
Waste Disposal
Careful incineration
Triethylaluminum Preparation Products And Raw materials
Raw materials
Preparation Products
1of3
chevron_right| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Alfa Aesar | 1 800 209 7001 | Maharashtra, India | 6913 | 58 | Inquiry |
| Srinivasa Labs | 09515114455 | Hyderabad, India | 113 | 58 | Inquiry |
| manikanta fine chem | 08048250466Ext 254 | Hyderabad, India | 133 | 58 | Inquiry |
| VASIN BIO LIFE SCIENCES PVT LTD | 9491644688 | Hyderabad, India | 2096 | 58 | Inquiry |
| Almas Life Sciences | 09441456585 | Hyderabad, India | 68 | 58 | Inquiry |
| Vinsa Pharmaceuticals | 91-9246578585 | Andhra Pradesh, India | 32 | 58 | Inquiry |
| Dalian Richfortune Chemicals Co., Ltd | 0411-84820922 8613904096939 | China | 304 | 57 | Inquiry |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | China | 21675 | 55 | Inquiry |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418679 +86-18949832763 | China | 2989 | 55 | Inquiry |
| career henan chemical co | +86-0371-86658258 +8613203830695 | China | 29900 | 58 | Inquiry |
97-93-8(Triethylaluminum)Related Search:
1of4
chevron_right




