Imatinib

- CAS No.
- 152459-95-5
- Chemical Name:
- Imatinib
- Synonyms
- Imatinib Mesilate;matinib;4-(4-METHYL-PIPERAZIN-1-YLMETHYL)-N-[4-METHYL-3-(4-PYRIDIN-3-YL-PYRIMIDIN-2-YLAMINO)-PHENYL]-BENZAMIDE;IMA-3;Veenat;ST-1571;CS-1955;IMATINIB;lmatinib;CGP057148B
- CBNumber:
- CB7370890
- Molecular Formula:
- C29H31N7O
- Molecular Weight:
- 493.6
- MOL File:
- 152459-95-5.mol
- Modify Date:
- 2024/9/11 18:44:42
| Melting point | 208-210°C (dec.) |
|---|---|
| Density | 1?+-.0.06 g/cm3(Predicted) |
| storage temp. | Keep in dark place,Sealed in dry,Store in freezer, under -20°C |
| solubility | DMSO (Slightly, Heated), Methanol (Slightly, Heated) |
| pka | pKa1 8.07; pKa2 3.73; pKa3 2.56; pKa4 1.52(at 25℃) |
| form | Solid |
| color | White to Pale Beige |
| Merck | 14,4902 |
| CAS DataBase Reference | 152459-95-5(CAS DataBase Reference) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS07,GHS08 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H315-H319-H335-H361fd | |||||||||
| Precautionary statements | P261-P271-P280 | |||||||||
| Safety Statements | 24/25 | |||||||||
| RTECS | CV5585673 | |||||||||
| HS Code | 29339900 | |||||||||
| NFPA 704 |
|
Imatinib price More Price(2)
Imatinib Chemical Properties,Uses,Production
Chemical Properties
Orange Solid
Uses
Imatinib impurity.
Indications
Bcr-Abl inhibitor imatinib (Gleevec(R), Novartis) was approved in 2001 by the FDA. Although fasudil was approved in 1995, imatinib is widely perceived as the first approved SMKI mainly owing to the fact that fasudil's kinase inhibitory mechanism was unknown at the time of approval, and efforts to gain approval of fasudil have been unsuccessful in the United States and Europe.
The field of kinase inhibitor development has evolved rapidly since the approval of imatinib. Some of the key discoveries and events include (i) the discovery of MAPK/ERK inhibitors, for example, CI-1040 (PD184352), as the first series of type III inhibitors in 2003; (ii) the approval of first dual tyrosine kinase and serine/threonine kinase inhibitor sorafenib in 2005; (iii) the description of the first series of allosteric type IVkinase inhibitor, that is,GNF-2 and analogues that inhibit Bcr–Abl through an allosteric non-ATP-competitivemode, by Gray and coworkers in 2006; (iv) the approval of the first type III inhibitor trametinib in 2013; (v) the approval of the first covalent kinase inhibitors, afatinib and ibrutinib, in 2013; and (vi) the approval of the first lipid kinase inhibitor, that is, the PI3K inhibitor idelalisib, in 2014.
By December 2016, kinase inhibitor drug discovery can leverage the structures of over 200 human kinases and 5000 kinases of all types, over 1 million publications, clinical data from more than 200 molecules currently in phase I–III trials, and post-marketing results from the approved 38 drugs.
General Description
non-receptor tyrosine kinase|
Treatment: CML, ALL, GIST
Oral bioavailability = 98%
Elimination half-life = 20 h
Protein binding = 95%
Mode of action
Imatinib binds close to the ATP-binding site of BCR-ABL and locks it in a closed inactive conformation which excludes ATP. This shuts down its sustained signaling, thus blocking leukemic cell growth.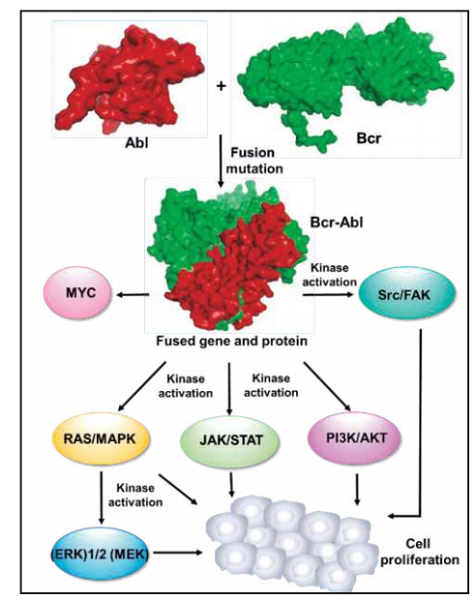
Imatinib Preparation Products And Raw materials
Raw materials
1of2
chevron_rightPreparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| TAGOOR LABORATORIES PVT LTD | +919700187008 | AndhraPradesh, India | 93 | 58 | Inquiry |
| Urantiis Pharmaceuticals Private Limited | +918790703567 | AndhraPradesh, India | 80 | 58 | Inquiry |
| Rampex Labs Private Limited | +91 9168074433 | Telangana, India | 71 | 58 | Inquiry |
| SGMR PHARMACEUTICALS PVT LTD | +91-9032001889 +91-9032001889 | Telangana, India | 83 | 58 | Inquiry |
| GSV speciality chemicals | +91-91823634932 +91-91823634932 | Hyderabad, India | 135 | 58 | Inquiry |
| J S LABS | +91-7330612784 +91-7330612784 | Tamil Nadu, India | 160 | 58 | Inquiry |
| Lorven therapeutics Pvt Ltd | +91-8884110005 +91-8884110005 | Hyderabad, India | 110 | 58 | Inquiry |
| SVK Laboratories Pvt Ltd | +91-40-23095079 +91-9177729915 | Telangana, India | 89 | 58 | Inquiry |
| HRV Global Life Sciences | +91-9820219686 +91-9820219686 | Telangana, India | 379 | 58 | Inquiry |
| ALS INDIA LIFE SCIENCES | +91-8977036379 +91-8977036379 | Hyderabad, India | 1879 | 58 | Inquiry |
152459-95-5(Imatinib)Related Search:
1of4
chevron_right




