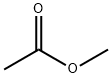
Methyl acetate
- CAS No.
- 79-20-9
- Chemical Name:
- Methyl acetate
- Synonyms
- ACETIC ACID METHYL ESTER;CH3COOCH3;methylethanoate;Acetate de methyle;Metile;Devoton;Tereton;Methylacetat;Methyl ethanoate;methyle(acetatede)
- CBNumber:
- CB9167443
- Molecular Formula:
- C3H6O2
- Molecular Weight:
- 74.08
- MOL File:
- 79-20-9.mol
- MSDS File:
- SDS
- Modify Date:
- 2024/7/1 10:46:47
| Melting point | -98 °C (lit.) |
|---|---|
| Boiling point | 57-58 °C (lit.) |
| Density | 0.934 g/mL at 25 °C |
| vapor density | 2.55 (vs air) |
| vapor pressure | 165 mm Hg ( 20 °C) |
| refractive index |
n |
| FEMA | 2676 | METHYL ACETATE |
| Flash point | 3.2 °F |
| storage temp. | no restrictions. |
| solubility | 250g/l |
| form | Solution |
| color | Clear colorless to slightly pale yellow |
| Relative polarity | 0.253 |
| Odor | Slightly acrid, sweet; fragrant. |
| Odor Threshold | 1.7ppm |
| explosive limit | 3.1-16%(V) |
| Odor Type | ethereal |
| Water Solubility | 250 g/L (20 ºC) |
| λmax |
λ: 255 nm Amax: 1.0 λ: 275 nm Amax: 0.1 λ: 300 nm Amax: 0.01 |
| JECFA Number | 125 |
| Merck | 14,6008 |
| BRN | 1736662 |
| Henry's Law Constant | 0.90 at 20.00 °C, 1.56 at 30.00 °C (headspace-GC, Hovorka et al., 2002) |
| Exposure limits | TLV-TWA 200 ppm (~610 mg/m3) (ACGIH, MSHA, and OSHA); TLV-STEL 250 ppm (~760 mg/m3) (ACGIH); IDLH 10,000 ppm (NIOSH). |
| Dielectric constant | 7.3(20℃) |
| Stability | Stable. Extremely flammable - readily forms explosive mixtures with air. Note low flash point and wide explosion limits. Incompatible with strong oxidizing agents, strong bases, strong acids, nitrates. May be moisture sensitive. |
| LogP | 0.18 at 20℃ |
| CAS DataBase Reference | 79-20-9(CAS DataBase Reference) |
| NIST Chemistry Reference | Acetic acid, methyl ester(79-20-9) |
| EPA Substance Registry System | Methyl acetate (79-20-9) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS02,GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H225-H319-H336 | |||||||||
| Precautionary statements | P210-P233-P240-P241-P242-P305+P351+P338 | |||||||||
| Hazard Codes | F,Xi | |||||||||
| Risk Statements | 11-36-66-67 | |||||||||
| Safety Statements | 16-26-29-33 | |||||||||
| RIDADR | UN 1231 3/PG 2 | |||||||||
| OEB | A | |||||||||
| OEL | TWA: 200 ppm (610 mg/m3), STEL: 250 ppm (760 mg/m3) | |||||||||
| WGK Germany | 1 | |||||||||
| RTECS | AI9100000 | |||||||||
| Autoignition Temperature | 936 °F | |||||||||
| TSCA | Yes | |||||||||
| HS Code | 2915 39 00 | |||||||||
| HazardClass | 3 | |||||||||
| PackingGroup | II | |||||||||
| Toxicity | LD50 orally in Rabbit: > 5000 mg/kg LD50 dermal Rat > 2000 mg/kg | |||||||||
| IDLA | 3,100 ppm [10% LEL] | |||||||||
| NFPA 704 |
|
Methyl acetate price More Price(28)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | W267600 | Methyl acetate ≥98%, FG | 79-20-9 | 1SAMPLE | ₹5141.88 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | W267619 | Methyl acetate natural, 98%, FG | 79-20-9 | 1SAMPLE-K | ₹5347.55 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | W267600 | Methyl acetate ≥98%, FG | 79-20-9 | 1KG | ₹8194.53 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | W267600 | Methyl acetate ≥98%, FG | 79-20-9 | 5KG | ₹12492.05 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | W267619 | Methyl acetate natural, 98%, FG | 79-20-9 | 1KG | ₹23197.98 | 2022-06-14 | Buy |
Methyl acetate Chemical Properties,Uses,Production
Description
Methyl acetate, also known as MeOAc , acetic acid methyl ester or methyl ethanoate, is a carboxylate ester with the formula CH3COOCH3. It is a flammable liquid with a characteristically pleasant smell reminiscent of some glues and nail polish removers. Methyl acetate is occasionally used as a solvent, being weakly polar and lipophilic, but its close relative ethyl acetate is a more common solvent being less toxic and less soluble in water. Methyl acetate has a solubility of 25% in water at room temperature. At elevated temperature its solubility in water is much higher. Methyl acetate is not stable in the presence of strong aqueous bases or aqueous acids. Methyl acetate is VOC exempt.
Chemical Properties
Methyl acetate has a pleasant, fruity odor and slightly bitter flavor. May be prepared by boiling acetic acid and methanol in the presence of acid catalysts; or by heating methanol with an excess carbon monoxide under pressure in the presence of a catalyst (phosphoric acid, cobalt salts).
Methyl acetate occurs naturally in low concentrations in mint, fungus,grapes, banana, coffee (Furia and Bellanca, 1975) and is a volatile constituent of nectarines (Takeoka et al., 1988). It is also present in some distilled alcoholic beverages (Shimoda et al., 1993). It is produced industrially via the carbonylation of methanol as a byproduct of acetic acid production or by esterification of acetic acid with methanol in the presence of strong acid such as sulfuric acid.
Physical properties
Colorless liquid with a pleasant odor. An odor threshold concentration of 48 ppbv was reported by Nagata and Takeuchi (1990). Cometto-Mu?iz and Cain (1991) reported an average nasal pungency threshold concentration of 112,500 ppmv.
Occurrence
Reported found in apple, banana, sweet and sour cherry, tangerine juice, black currants, guava, grapes, melon, peach, pear, pineapple, strawberry, cabbage, tomato, clove bud, peppermint oil, vinegar, bread, cheeses, butter, yogurt, beef, beer, cognac, rum, whiskies, cider, sherry, grape wines, cocoa, coffee, filbert, peanut, honey, soybean, olive, passion fruit, fruit brandies, fig, gin, kiwifruit, clary sage, arrack and nectarine.
Uses
Methyl acetate is used as a solvent forlacquers, resins, oils, and nitrocellulose; inpaint removers; as a flavoring agent; and inthe manufacture of artificial leather.
Preparation
Methyl acetate is produced industrially via the carbonylation of methanol as a byproduct of the production of acetic acid.Methyl acetate also arises by esterification of acetic acid with methanol in the presence of strong acids such as sulfuric acid, this production process is famous because of Eastman Kodak's intensified process using a reactive distillation.
2–1-Reactions
In the presence of strong bases such as sodium hydroxide or strong acids such as hydrochloric acid or sulfuric acid it is hydrolyzed back into methanol and acetic acid, especially at elevated temperature. The conversion of methyl acetate back into its components, by an acid , is a first-order reaction with respect to the ester. The reaction of methyl acetate and a base, for example sodium hydroxide, is a second-order reaction with respect to both reactants.
3-Applications
A major use of methyl acetate is as a volatile low toxicity solvent in glues, paints, and nail polish removers. Acetic anhydride is produced by carbonylation of methyl acetate in a process that was inspired by the Monsanto acetic acid synthesis.
Definition
ChEBI: Methyl acetate is an acetate ester resulting from the formal condensation of acetic acid with methanol. A low-boiling (57°C) colourless, flammable liquid, it is used as a solvent for many resins and oils. It has a role as a polar aprotic solvent, a fragrance and an EC 3.4.19.3 (pyroglutamyl-peptidase I) inhibitor. It is an acetate ester, a methyl ester and a volatile organic compound.
Application
Methyl acetate may be used for the preparation of fatty acid methyl esters and triacetin from rapeseed oil via non-catalytic trans-esterification reaction under super-critical conditions.
Methyl acetate may be used in the following:
As acyl acceptor in the preparation of biodiesel.
Synthesis of ethanol.
Preparation of n-butyl acetate, via transesterification reaction with n-butanol in the presence of acidic catalysts.
It may also be used as a precursor in the synthesis of the following:
acetic anhydride
methyl acrylate
vinyl acetate
ethyl amide
General Description
Methyl acetate appears as a clear colorless liquid with a fragrant odor. Moderately toxic. Flash point 14 °F. Vapors heavier than air.
Air & Water Reactions
Highly flammable. Water soluble.
Reactivity Profile
Methyl acetate presents a fire or explosion hazard when exposed to strong oxidizing agents. Emits irritating fumes and acrid smoke when heated to decomposition, [Lewis, 3rd ed., 1993, p. 826]. Methyl acetate reactivity is consistent with other compounds of the ester group.
Hazard
Flammable, dangerous fire and explosion risk, explosive limits in air 3–16%. Irritant to respiratory tract. Headache, dizziness, nausea, eye damage (degeneration of ganglion cells in the retina).
Health Hazard
(Very similar to those of methyl alcohol, which constitutes 20% of commercial grade.) Inhalation causes headache, fatigue, and drowsiness; high concentrations can produce central nervous system depression and optic nerve damage. Liquid irritates eyes and may cause defatting and cracking of skin. Ingestion causes headache, dizziness, drowsiness, fatigue; may cause severe eye damage.
Fire Hazard
Behavior in Fire: Vapor is heavier than air and may travel a considerable distance to a source of ignition and flash back.
Safety Profile
Moderately toxic by several routes. A human systemic irritant by inhalation. A moderate skin and eye irritant. Mutation data reported. Dangerous fire hazard when exposed to heat, flame, or oxidizers. Moderate explosion hazard when exposed to heat or flame. When heated to decomposition it emits acrid smoke and fumes. See also ESTERS.
Potential Exposure
Methyl acetate is used as a solvent in lacquers and paint removers; and as an intermediate in pharmaceutical manufacture.
Environmental Fate
Photolytic. A rate constant of 2.00 x 10-13 cm3/molecule?sec was reported for the reaction of
methyl acetate and OH radicals in aqueous solution (Wallington et al., 1988b).
Chemical/Physical. Slowly hydrolyzes in water yielding methyl alcohol and acetic acid
(NIOSH, 1997). The estimated hydrolysis half-life in water at 25 °C and pH 7 is 2.5 yr (Mabey and
Mill, 1978).
At an influent concentration of 1,030 mg/L, treatment with GAC resulted in an effluent
concentration of 760 mg/L. The adsorbability of the carbon used was 54 mg/g carbon (Guisti et
al., 1974).
Shipping
UN1231 Methyl acetate, Hazard Class: 3; Labels: 3-Flammable liquid.
Purification Methods
Methanol in methyl acetate can be detected by measuring its solubility in water. At 20o, the solubility of methyl acetate in water is ca 35g per 100mL, but 1% MeOH confers complete miscibility. Methanol can be removed by conversion to methyl acetate, by refluxing for 6hours with acetic anhydride (85mL/L), followed by fractional distillation. Acidic impurities can be removed by shaking with anhydrous K2CO3 and distilling. An alternative treatment is with acetyl chloride, followed by washing with concentrated NaCl and drying with CaO or MgSO4. (Solid CaCl2 cannot be used because it forms a crystalline addition compound.) Distillation from copper stearate destroys peroxides. Free alcohol or acid can be eliminated from methyl acetate by shaking with strong aqueous Na2CO3 or K2CO3 (three times), then with aqueous 50% CaCl2 (three times), saturated aqueous NaCl (twice), drying with K2CO3 and distilling it from P2O5. [Beilstein 2 IV 122.]
Incompatibilities
Vapor may form explosive mixture with air. A Strong reducing agent. Incompatible water, acids, nitrates, strong oxidizers; alkalis. Attacks some plastics. Attacks many metals in presence of water. Reacts slowly with water, forming acetic acid and methanol. Decomposes in heat; on contact with air, bases, strong oxidizers; UV-light; possible fire and explosion hazard
Waste Disposal
Dissolve or mix the material with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber. All federal, state, and local environmental regulations must be observed.
Methyl acetate Preparation Products And Raw materials
Raw materials
Preparation Products
1of4
chevron_right| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Bhavika Chemicals Corporation | +91-9867153307 +91-9867153307 | Maharashtra, India | 31 | 58 | Inquiry |
| UNILOSA INTERNATINAL PRIVATE LIMITED | +91-9999069917 +91-9999069917 | New Delhi, India | 152 | 58 | Inquiry |
| JSK Chemicals | +919879767970 | Gujarat, India | 3756 | 58 | Inquiry |
| Beetachem Industries | +91-9029419019 +91-9757110215 | Maharashtra, India | 12 | 58 | Inquiry |
| Sontara Organo Industries | +919423554439 | Maharashtra, India | 46 | 58 | Inquiry |
| Ruchi Menthol Pvt Ltd | +91-9323165363 +91-9324144305 | Maharashtra, India | 22 | 58 | Inquiry |
| HiMedia Laboratories | 91-22-61471919 | Maharashtra, India | 1843 | 58 | Inquiry |
| Associated Agencies | 91-22-28231752 | Maharashtra, India | 29 | 58 | Inquiry |
| Arnish Laborates Private Limited | 08048957357 | Mumbai, India | 204 | 58 | Inquiry |
| The Himatlal Group | 91-22-22870526 | Maharashtra, India | 22 | 58 | Inquiry |
Related articles
- Is Methyl acetate safety
- In the body, methyl acetate is hydrolyzed into methanol and acetic acid. It causes narcosis in animals exposed to high concent....
- Jul 1,2024
- The application of Methyl acetate
- Methyl acetate appears as a clear colorless liquid with a fragrant odor. Moderately toxic. Flash point 14°F. Vapors heavier th....
- Jul 5,2022
79-20-9(Methyl acetate)Related Search:
1of4
chevron_right




