Methyl formate
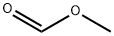
- CAS No.
- 107-31-3
- Chemical Name:
- Methyl formate
- Synonyms
- HCOOCH3;METHYL METHANOATE;Methyl formate analytical standard;Metil;formiatedemethyle;formiatedemethyle(french);Methyl forMate (C1:0) Solution;METHYL FORMATE FOR SYNTHESIS 1 L;METHYL FORMATE FOR SYNTHESIS 100 ML;acid-Me
- CBNumber:
- CB9106448
- Molecular Formula:
- C2H4O2
- Molecular Weight:
- 60.05
- MOL File:
- 107-31-3.mol
- MSDS File:
- SDS
- Modify Date:
- 2024/7/3 13:03:20
| Melting point | -100 °C (lit.) |
|---|---|
| Boiling point | 32-34 °C (lit.) |
| Density | 0.974 g/mL at 20 °C (lit.) |
| vapor density | 2.1 (vs air) |
| vapor pressure | 32.91 psi ( 55 °C) |
| refractive index |
n |
| Flash point | −16 °F |
| storage temp. | Store below +30°C. |
| solubility | 300g/l, 25% soluble in water, miscible with alcohol, Propylene glycol, Glycerin and oils. |
| form | Liquid |
| color | Clear colorless |
| PH | 4-5 (200g/l, H2O, 20℃) |
| Odor | Pleasant; agreeable. |
| explosive limit | 5-23%(V) |
| Odor Threshold | 130ppm |
| Odor Type | fruity |
| Water Solubility | 300 G/L (20 ºC) |
| λmax |
λ: 259 nm Amax: 1.00 λ: 260 nm Amax: 0.70 λ: 265 nm Amax: 0.20 λ: 270 nm Amax: 0.04 λ: 310-400 nm Amax: 0.01 |
| Merck | 14,6077 |
| BRN | 1734623 |
| Henry's Law Constant | 0.90 at 5.00 °C, 1.18 at 10.00 °C, 1.51 at 15.00 °C, 1.91 at 20.00 °C, 2.36 at 25.00 °C (column stripping-UV, Kutsuna et al., 2005) |
| Exposure limits | TLV-TWA 100 ppm (~250 mg/m3) (ACGIH, MSHA, and OSHA); TLV-STEL 150 ppm (~375 mg/m3) (ACGIH); IDLH 5000 ppm (NIOSH). |
| Dielectric constant | 8.5(20℃) |
| Stability | Stable. Extremely flammable. Readily forms explosive mixtures with air. Note low flash point and very wide explosion limits. Incompatible with oxidizing agents. |
| LogP | -0.21 at 25℃ |
| CAS DataBase Reference | 107-31-3(CAS DataBase Reference) |
| NIST Chemistry Reference | Methyl formate(107-31-3) |
| EPA Substance Registry System | Methyl formate (107-31-3) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS02,GHS06,GHS08 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H224-H301+H311+H331-H319-H335-H370 | |||||||||
| Precautionary statements | P210-P280-P301+P310+P330-P302+P352+P312-P304+P340+P311-P403+P233 | |||||||||
| Hazard Codes | F+,Xn | |||||||||
| Risk Statements | 12-20/22-36/37 | |||||||||
| Safety Statements | 9-16-24-26-33 | |||||||||
| OEB | A | |||||||||
| OEL | TWA: 100 ppm (250 mg/m3), STEL: 150 ppm (375 mg/m3) | |||||||||
| RIDADR | UN 1243 3/PG 1 | |||||||||
| WGK Germany | 1 | |||||||||
| RTECS | LQ8925000 | |||||||||
| Autoignition Temperature | 842 °F | |||||||||
| TSCA | Yes | |||||||||
| HS Code | 2915 13 00 | |||||||||
| HazardClass | 3 | |||||||||
| PackingGroup | I | |||||||||
| Toxicity | LD50 orally in Rabbit: 1500 mg/kg LD50 dermal Rat > 4000 mg/kg | |||||||||
| IDLA | 4,500 ppm | |||||||||
| NFPA 704 |
|
Methyl formate price More Price(19)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | M46837 | Methyl formate reagent grade, 97% | 107-31-3 | 1L | ₹3409.88 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | W519103 | Methyl formate ≥95% | 107-31-3 | 1SAMPLE | ₹5141.88 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 8.00889 | Methyl formate for synthesis | 107-31-3 | 100ML | ₹5360 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | M46837 | Methyl formate reagent grade, 97% | 107-31-3 | 4X1L | ₹6798.1 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 8.00889 | Methyl formate for synthesis | 107-31-3 | 1L | ₹8470 | 2022-06-14 | Buy |
Methyl formate Chemical Properties,Uses,Production
Description
Methyl Formate, also known as methyl methanoate, is the methyl ester of formic acid. The simplest example of an ester, it is a clear liquid with an ethereal odour, high vapor pressure, and low surface tension. It is an aromatic compound found in apples (Neubeller and Buchloh, 1986), and was identified as a volatile constituent in brewed, roasted, and dried coffee (Lovell et al., 1980); Methyl formate is used primarily to manufacture formamide, dimethylformamide, and formic acid. It is also used as a solvent for quick-drying finishes such as lacquers and in organic synthesis.
Chemical Properties
Methyl formate is a colorless liquid with a pleasant odor. Its solubility in water is 230 g/l at 25 °C (Riddick et al., 1985), but it reacts slowly with water to form formic acid and methyl alcohol (DOT, 1984). It is soluble in ether, chloroform, and is miscible with ethanol (Lide, 2000).
Physical properties
Clear, colorless, mobile liquid with a pleasant, etheral odor. An odor threshold concentration of 130 ppmv was reported by Nagata and Takeuchi (1990).
Uses
Methyl formate is used as a fumigant, as alarvicide for food crops, and as a solvent forcellulose acetate.
Production Methods
In the laboratory, methyl formate can be produced by the condensation reaction of methanol and formic acid, as follows:
HCOOH + CH3OH → HCOOCH3 + H2O
Industrial methyl formate, however, is usually produced by the combination of methanol and carbon monoxide (carbonylation) in the presence of a strong base, such as sodium methoxide :
CH3OH + CO → HCOOCH3
This process, practiced commercially by BASF among other companies gives 96 % selectivity toward methyl formate, although it can suffer from catalyst sensitivity to water, which can be present in the carbon monoxide feedstock, commonly derived from synthesis gas. Very dry carbon monoxide is, therefore, an essential requirement.
Definition
ChEBI: Methyl formate is a formate ester resulting from the formal condensation of formic acid with methanol. A low-boiling (31.5 ℃) colourless, flammable liquid, it has been used as a fumigant and larvicide for tobacco and food crops. It has a role as a polar aprotic solvent, a fumigant, an insecticide and a refrigerant. It is a formate ester, a methyl ester and a volatile organic compound. It is functionally related to a methanol.
General Description
A clear colorless liquid with an agreeable odor. Flash point -27°F. Less dense than water Vapors heavier than air.
Air & Water Reactions
Highly flammable. Water soluble. Reacts slowly with water to give formic acid, a corrosive material, and methanol, a flammable liquid. Both products are dissolved in the water.
Reactivity Profile
Methyl formate reacts with acids to liberate heat along with alcohols and acids. Strong oxidizing acids may cause a vigorous reaction that is sufficiently exothermic to ignite the reaction products. Heat is also generated with caustic solutions. Flammable hydrogen is generated by mixing with alkali metals and hydrides.
Hazard
Flammable, dangerous fire and explosionrisk, explosive limits in air 5.9–20%. Eye, upperand lower respiratory tract irritant.
Health Hazard
Methyl formate is a moderately toxic com pound affecting eyes, respiratory tract, andcentral nervous system. It is an irritant tothe eyes, nose, and lungs. Exposure to highconcentrations of its vapors in air may pro duce visual disturbances, irritations, narcoticeffects, and respiratory distress in humans.Such effects may be manifested at a 1-hourexposure to about 10,000-ppm concentration.Cats died of pulmonary edema from 2-hourexposure to this concentration
The acute oral toxicity of methyl formatewas low in test subjects. The symptoms werenarcosis, visual disturbances, and dyspnea.An oral LD50 value in rabbit is in the range1600 mg/kg..
Fire Hazard
Behavior in Fire: Vapor is heavier than air and may travel considerable distance to a source of ignition and flash back.
Safety Profile
Moderately toxic by ingestion. Inhalation of vapor can cause irritation to nasal passages and conjunctiva, optic neuritis, narcosis, retching, and death from pulmonary irritation. Industrial fatalities have occurred only with exposure to high concentrations. Flammable liquid. Very dangerous fire hazard when exposed to heat or flame; can react vigorously with oxidizing materials. Explosive in the form of vapor when exposed to heat or flame. Reacts with methanol + sodium methoxide to form an explosive product. To fight fire, use alcohol foam, CO2, dry chemical. When heated to decomposition it emits acrid smoke and irritating fumes.
Potential Exposure
Methyl formate is used as a solvent; as an intermediate in pharmaceutical manufacture; and as a fumigant
Environmental Fate
Photolytic. Methyl formate, formed from the irradiation of dimethyl ether in the presence of
chlorine, degraded to carbon dioxide, water, and small amounts of formic acid. Continued
irradiation degraded formic acid to carbon dioxide, water, and hydrogen chloride (Kallos and Tou,
1977; Good et al., 1999).
A rate constant of 2.27 x 10-12 cm3/molecule?sec was reported for the reaction of methyl formate
and OH radicals in the atmosphere (Atkinson, 1989).
Chemical/Physical. Hydrolyzes slowly in water forming methanol and formic acid (NIOSH,
1997). Hydrolysis half-lives reported at 25 °C: 0.91 h at pH 9, 9.1 h at pH 8, 2.19 d at pH 7, and
21.9 d at pH 6 (Mabey and Mill, 1978).
Purification Methods
Wash the formate with strong aqueous Na2CO3, dry it with solid Na2CO3 and distil it from P2O5. (Procedure removes free alcohol or acid.) [Beilstein 2 IV 20.]
Incompatibilities
May form explosive mixture with air. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides. Reacts slowly with water to form methanol and formic acid. Contact with water, steam releases formic acid. Compounds of the carboxyl group react with all bases, both inorganic and organic (i.e., amines) releasing substantial heat, water and a salt that may be harmful. Incompatible with arsenic compounds (releases hydrogen cyanide gas), diazo compounds, dithiocarbamates isocyanates, mercaptans, nitrides, and sulfides (releasing heat, toxic, and possibly flammable gases), thiosulfates and dithionites (releasing hydrogen sulfate and oxides of sulfur)
Waste Disposal
Incineration; atomizing in a suitable combustion chamber.
References
Lee, Jae S., J. C. Kim, and Y. G. Kim. "Methyl formate as a new building block in C1 chemistry." Applied Catalysis 57.1 (1990): 1-30.
Handa, Yash Paul, et al. "Insulating Thermoplastic Foams Made With Methyl Formate-Based Blowing Agents." (2006).
Methyl formate Preparation Products And Raw materials
Raw materials
Preparation Products
1of7
chevron_right| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| JSK Chemicals | +919879767970 | Gujarat, India | 3756 | 58 | Inquiry |
| Beetachem Industries | +91-9029419019 +91-9757110215 | Maharashtra, India | 12 | 58 | Inquiry |
| Gujarat Narmada Valley Fertilizers Chemicals Ltd | +91-2642-247001 +91-9974060154 | Gujarat, India | 11 | 58 | Inquiry |
| Rashtriya Chemicals and Fertilizers Limited | +91-9969062114 +91-9139357099 | Maharashtra, India | 13 | 58 | Inquiry |
| TCI Chemicals (India) Pvt. Ltd. | 1800 425 7889 | New Delhi, India | 6778 | 58 | Inquiry |
| Otto Chemie Pvt. Ltd. | +91 9820041841 | Mumbai, India | 5873 | 58 | Inquiry |
| Alfa Aesar | 1 800 209 7001 | Maharashtra, India | 6913 | 58 | Inquiry |
| LOBA CHEMIE PVT.LTD. | 91-22-6663 6699 | Mumbai, India | 3077 | 58 | Inquiry |
| Central Drug House(P) Ltd. | 91-11-49404040 | New Delhi, India | 6160 | 58 | Inquiry |
| CLEARSYNTH LABS LTD. | +91-22-45045900 | Hyderabad, India | 6351 | 58 | Inquiry |
107-31-3(Methyl formate)Related Search:
1of4
chevron_right




