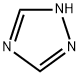
1,2,4-Triazole
- CAS No.
- 288-88-0
- Chemical Name:
- 1,2,4-Triazole
- Synonyms
- TRIAZOLE;1H-1,2,4-TRIAZOLE;4H-1,2,4-triazole;1,2,4-1H-TRIAZOLE;1,2,4-Triazol;PYRRODIAZOLE;1H-1,2,4-Triazol;1,2,4-Triazole,99%;TA-4;TAR4
- CBNumber:
- CB2277901
- Molecular Formula:
- C2H3N3
- Molecular Weight:
- 69.07
- MOL File:
- 288-88-0.mol
- MSDS File:
- SDS
- Modify Date:
- 2024/8/21 22:41:43
| Melting point | 119-121 °C (lit.) |
|---|---|
| Boiling point | 260 °C (lit.) |
| Density | 1.15 g/cm3 (130℃) |
| vapor pressure | 0.215Pa at 20℃ |
| refractive index | 1.4854 (estimate) |
| Flash point | 140 °C |
| storage temp. | Store below +30°C. |
| solubility | 547g/l |
| form | Crystalline Powder and Flakes |
| pka | 2.27(at 20℃) |
| color | White |
| PH | 8 (10g/l, H2O, 20℃) |
| Water Solubility | 1250 g/L (20 ºC) |
| Merck | 14,9605 |
| BRN | 104767 |
| InChIKey | NSPMIYGKQJPBQR-UHFFFAOYSA-N |
| LogP | -0.76 at 25℃ |
| CAS DataBase Reference | 288-88-0(CAS DataBase Reference) |
| NIST Chemistry Reference | 1H-1,2,4-Triazole(288-88-0) |
| EPA Substance Registry System | 1,2,4-Triazole (288-88-0) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS07,GHS08 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H302-H319-H361d | |||||||||
| Precautionary statements | P201-P202-P264-P301+P312-P305+P351+P338-P308+P313 | |||||||||
| Hazard Codes | Xn,Xi,C | |||||||||
| Risk Statements | 22-36-63-34 | |||||||||
| Safety Statements | 36/37-45-36/37/39-27-26 | |||||||||
| WGK Germany | 2 | |||||||||
| RTECS | XZ3806000 | |||||||||
| Hazard Note | Irritant | |||||||||
| TSCA | Yes | |||||||||
| HS Code | 29339990 | |||||||||
| Toxicity | LD50 orally in Rabbit: 1650 mg/kg LD50 dermal Rabbit 3129 - 4200 mg/kg | |||||||||
| NFPA 704 |
|
1,2,4-Triazole price More Price(15)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | T46108 | 1,2,4-Triazole 98% | 288-88-0 | 25G | ₹2749.55 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | T46108 | 1,2,4-Triazole 98% | 288-88-0 | 100G | ₹7512.55 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | T46108 | 1,2,4-Triazole 98% | 288-88-0 | 500G | ₹22072.18 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 8.08388 | 1,2,4-Triazole for synthesis | 288-88-0 | 25G | ₹3340 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 8.08388 | 1,2,4-Triazole for synthesis | 288-88-0 | 100G | ₹10610 | 2022-06-14 | Buy |
1,2,4-Triazole Chemical Properties,Uses,Production
Chemical Properties
white crystalline powder and flakes
Uses
1,2,4-Triazole is present in a large variety of fungicides as C14-demethylase-inhibitors. In addition they are used in synthetic chemical reactions such as the design and preparation of ghrelin receptor ligand antagonists, like JMV 2959.
Definition
1,2,4-triazole is a chemical substance with molecular formula C2H3N3, molecular weight 69.07 and CAS No. 288-88-0. Colorless acicular crystal, soluble in water and ethanol. 1H-1,2,4-triazole is a 1,2,4-triazole. It is a tautomer of a 3H-1,2,4-triazole and a 4H-1,2,4-triazole.
Triazole is an aromatic five membered heterocyclic compound. Its unique structure is conducive to the formation of a variety of non covalent bonds and is easy to promote the binding of proteins, enzymes and receptors, thus showing a wide range of biological pharmacological activities, such as anticancer, antispasmodic, antibacterial, anti HIV. In particular, 1,2,3-triazole has strong thermal stability and acid-base stability, and is not sensitive to redox, hydrolysis and enzymatic degradation. It is often used as a pharmacodynamic group in drug synthesis.
Synthesis
Alkyl bromide and sodium azide were used as raw materials to prepare organic azides, and a series of 1,2,4-Triazole small molecules were prepared by click chemistry. The structures of the six products were characterized by 1H NMR, 13C NMR and IR. the products were expected targets. Furthermore, the effect of different substituent structure on the reaction was discussed. Especially the introduction of drug molecule ethisterone, which is expected to provide a certain experimental basis for the application of triazole and its derivatives in biomedicine and other fields.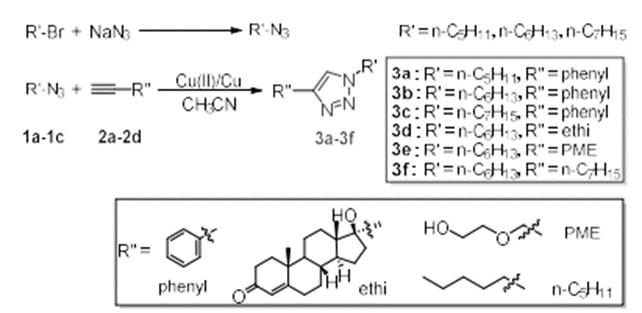 As shown in the figure, alkyl bromide and sodium azide were used as raw materials, N, N-dimethylformamide was used as solvent, and reacted at room temperature for 20 hours under the protection of nitrogen. After the reaction, the solvent was extracted, dried, filtered and spin evaporated to obtain alkyl azide compound 1.Put a certain proportion of alkyl azide 1 and alkynyl compound 2 into a round bottom flask, copper powder and copper acetate as catalysts, acetonitrile as solvent, and react at room temperature for 4 hours under the protection of nitrogen. After that, extract, dry, filter and spin the solvent, and purify by thin chromatography to obtain triazole compound 3.
As shown in the figure, alkyl bromide and sodium azide were used as raw materials, N, N-dimethylformamide was used as solvent, and reacted at room temperature for 20 hours under the protection of nitrogen. After the reaction, the solvent was extracted, dried, filtered and spin evaporated to obtain alkyl azide compound 1.Put a certain proportion of alkyl azide 1 and alkynyl compound 2 into a round bottom flask, copper powder and copper acetate as catalysts, acetonitrile as solvent, and react at room temperature for 4 hours under the protection of nitrogen. After that, extract, dry, filter and spin the solvent, and purify by thin chromatography to obtain triazole compound 3.
Purification Methods
Crystallise 1,2,4-triazole from EtOH, H2O, EtOAc (m 120.5-121o), or EtOH/*C6H6. The hydrochloride has m 170o, and the picrate has m 163-164o (from H2O or CHCl3). [Barszcz et al. J Chem Soc, Dalton Trans 2025 1986]. [Beilstein 26 H 13, 26 II 6, 26 III/IV 35.]
1,2,4-Triazole Preparation Products And Raw materials
Raw materials
1of2
chevron_rightPreparation Products
1of4
chevron_right| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| BTC pharm India | +918790379245 | AndhraPradesh, India | 1269 | 58 | Inquiry |
| GLR Innovations | +91 9891111994 | New Delhi, India | 4542 | 58 | Inquiry |
| ANJI BIOSCIENCES | +91-9000100077 +91-9000100077 | Hyderabad, India | 430 | 58 | Inquiry |
| GRINDLAYS PHARMACEUTICALS PRIVATE LIMITED | +91-91-22-25278801 +91-2225278801 | Mumbai, India | 83 | 58 | Inquiry |
| Rsum Industries Pvt. Ltd. | +91-91062685255 +91-91062685255 | New Delhi, India | 29 | 58 | Inquiry |
| RIDDHI PHARMA | +91 7874046933 | Gujarat, India | 43 | 58 | Inquiry |
| BTC pharm India | +918790379245 | Telangana, India | 145 | 58 | Inquiry |
| MOLECRAFT LIFE SCIENCES PVT LTD | +91 9581188881 | Telangana, India | 189 | 58 | Inquiry |
| SNECOFRi Pvt Ltd | +91-9032850129 +91-9032850129 | Telangana, India | 404 | 58 | Inquiry |
| RATNAMANI BIO CHEMICALS AND PHARMACEUTICALS PVT LTD | +91-9924133621 +91-9924133621 | Gujarat, India | 25 | 58 | Inquiry |
Related articles
- 1,2,4-Triazole: Overview, Antioxidant Activities and Toxicity
- 1,2,4-Triazole offers diverse pharmacological benefits, including antioxidant properties, but warrants caution due to toxicity....
- May 30,2024
- Synthesis, Properties, Chemical Reactivity of 1,2,4-Triazole
- 1,2,4-Triazole is a five-membered, π-excessive, aromatic nitrogen heterocycle, comprised of two carbon and three nitrogen ato....
- Feb 14,2022
- The role of 1,2,4-triazole
- Triazole is an aromatic five membered heterocyclic compound. Its unique structure is conducive to the formation of a variety o....
- Jan 27,2022
288-88-0(1,2,4-Triazole)Related Search:
1of4
chevron_right




