Sulindac
- CAS No.
- 38194-50-2
- Chemical Name:
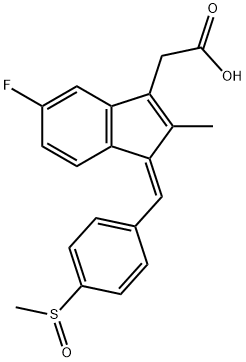
- Sulindac
- Synonyms
- Aclin;Sudac;Saldac;MK-231;ReuMyl;AFLODAC;Imbaral;mobilin;SULINOL;sulinac
- CBNumber:
- CB3740374
- Molecular Formula:
- C20H17FO3S
- Molecular Weight:
- 356.41
- MOL File:
- 38194-50-2.mol
- MSDS File:
- SDS
- Modify Date:
- 2024/7/2 8:55:06
| Melting point | 182-185°C |
|---|---|
| Boiling point | 581.6±50.0 °C(Predicted) |
| Density | 1.2581 (estimate) |
| storage temp. | Sealed in dry,Store in freezer, under -20°C |
| solubility | Very slightly soluble in water, soluble in methylene chloride, sparingly soluble in ethanol (96 per cent). It dissolves in dilute solutions of alkali hydroxides. |
| form | Solid |
| pka | pKa (25°) 4.7 |
| color | Light yellow to Brown |
| Water Solubility | Soluble in water, methanol, ethanol. |
| λmax | 327nm(0.05mol/L methanolic HCl)(lit.) |
| Merck | 14,8982 |
| CAS DataBase Reference | 38194-50-2(CAS DataBase Reference) |
| NIST Chemistry Reference | Sulindac(38194-50-2) |
| EPA Substance Registry System | 1H-Indene-3-acetic acid, 5-fluoro-2-methyl-1-[[4-(methylsulfinyl)phenyl]methylene]-, (1Z)- (38194-50-2) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS06,GHS08 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H301-H317-H334-H361 | |||||||||
| Precautionary statements | P261-P280-P284-P301+P330+P331+P310-P304+P340-P342+P311 | |||||||||
| Hazard Codes | Xn | |||||||||
| Risk Statements | 22-63-42/43 | |||||||||
| Safety Statements | - | |||||||||
| RIDADR | 3249 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | NK8226000 | |||||||||
| HazardClass | 6.1(b) | |||||||||
| PackingGroup | III | |||||||||
| HS Code | 29309090 | |||||||||
| NFPA 704 |
|
Sulindac price More Price(7)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | S8139 | Sulindac ≥98.0% | 38194-50-2 | 5G | ₹8854.85 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | S8139 | Sulindac ≥98.0% | 38194-50-2 | 25G | ₹34629.18 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | S4429 | Sulindac meets USP testing specifications | 38194-50-2 | 5G | ₹4611.45 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | S4429 | Sulindac meets USP testing specifications | 38194-50-2 | 25G | ₹13812.7 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | PHR3359 | Sulindac certified reference material, pharmaceutical secondary standard | 38194-50-2 | 500MG | ₹20643.28 | 2022-06-14 | Buy |
Sulindac Chemical Properties,Uses,Production
Description
Many non-
Chemical Properties
Yellow Crystalline Solid
Uses
Sulindac is a non-steroidal anti-inflammatory drug.
Indications
Sulindac (Clinoril) is chemically related to indomethacin and is generally used for the same indications. It is a prodrug that is metabolized to an active sulfide metabolite and an inactive metabolite. The most frequently reported side effects are GI pain, nausea, diarrhea, and constipation. The incidence of these effects is lower than for indomethacin, presumably because sulindac is a prodrug and thus the active metabolite is not highly concentrated at the gastric mucosa. As with indomethacin, a rather high incidence of CNS side effects (dizziness, headache) also occurs.
Definition
ChEBI: A monocarboxylic acid that is 1-benzylidene-1H-indene which is substituted at positions 2, 3, and 5 by methyl, carboxymethyl, and fluorine respectively, and in which the phenyl group of the benzylidene moiety is substituted at the ara position by a methylsulfinyl group. It is a prodrug for the corresponding sulfide, a non-steroidal anti-inflammatory drug, used particularly in the treatment of acute and chronic inflammatory conditions.
brand name
Clinoril (Merck). Aflodac (Biotekfarma, Italy), Algocetil (Francia Farm., Italy), Dorindac (Chibret, Portugal), Zirofalen (Farmalen, Greece).
General Description
Sulindac, (Z)-5-fluoro-2-methyl-1-([p-(methylsulfinyl)phenyl]methylene)-1H-indene-3-acetic acid (Clinoril), isan NSAID prodrug that contains a chiral sulfoxide moietybut is marketed as the racemate because it undergoes invivo reduction by the hepatic enzymes into its achiral, activemetabolite, methyl sulfide that exhibits potent andnonselective COX inhibition similar to indomethacin.
The parent sulfoxide has a plasma half-life of 8 hours, andthe active methyl sulfide metabolite is 16.4 hours. The morepolar and inactive sulfoxide is virtually the only form excretedinto the renal tubules, thus sulindac is believed to haveminimal nephrotoxicity associated with indomethacin. Thelong half-life of sulindac is caused by the extensive enterohepaticcirculation and reactivation of the inactive sulfoxideexcreted. Coadministration of aspirin is contraindicated becauseit considerably reduces the sulfide blood levels. Carefulmonitoring of patients with a history of ulcers is recommended.Gastric bleeding, nausea, diarrhea, dizziness, andother adverse effects have been noted with sulindac, but witha lower frequency than with aspirin. Sulindac is recommendedfor RA, OA, and ankylosing spondylitis.
Biological Activity
Prodrug. Metabolizes to sulindac sulfide, a cyclooxgenase inhibitor that represses ras signaling, and sulindac sulfone, an antitumor agent, following oral administration in vivo . Widely used anti-inflammatory agent.
Pharmacokinetics
Sulindac is well absorbed on oral administration (90%), reaches peak plasma levels within 2 to 4 hours, and being acidic (pKa = 4.5), is highly bound to serum proteins (93%). The metabolism of sulindac plays a major role in its actions, because all of the pharmacological activity is associated with its major metabolite. Sulindac is, in fact, a pro-drug, the sulfoxide function being reduced to the active sulfide metabolite. Sulindac is absorbed as the sulfoxide, which is not an inhibitor of prostaglandin biosynthesis in the GI tract. Prostaglandins exert a protective effect in the GI tract, and inhibition of their synthesis here leads to many of the GI side effects noted for most NSAIDs. Once sulindac enters the circulatory system, it is reduced to the sulfide, which is an inhibitor of prostaglandin biosynthesis in the joints. Thus, sulindac produces less GI side effects, such as bleeding, ulcerations, and so on, than indomethacin and many other NSAIDs. In addition, the active metabolite has a plasma half-life approximately twice that of the parent compound (~16 hours versus 8 hours), which favorably affects the dosing schedule. In addition to the sulfide metabolite, sulindac is oxidized to the corresponding sulfone, which is inactive. A minor product results from hydroxylation of the benzylidene function and the methyl group at the 2-position. Glucuronides of several metabolites also are found. Sulindac as well as the sulfide and the sulfone metabolites are all highly protein-bound. Despite the fact that the sulfide metabolite is a major activation product and is found in high concentration in human plasma, it is not found in human urine, perhaps because of its high degree of protein binding.
Clinical Use
Sulindac is indicated for long-term use in the treatment of rheumatoid arthritis, osteoarthritis, ankylosing spondylitis, and acute gouty arthritis. The usual maximum dosage is 400 mg/day, with starting doses recommended at 150 mg twice a day. It is recommended that sulindac be administered with food.
Side effects
Whereas the toxicity of sulindac is lower than that observed for indomethacin and other NSAIDs, the spectrum of adverse reactions is very similar. The most frequent side effects reported are associated with irritation of the GI tract (e.g., nausea, dyspepsia, and diarrhea), although these effects generally are mild. Effects on the CNS (e.g., dizziness and headache) are less common. Dermatological effects are less frequently encountered.
Sulindac Preparation Products And Raw materials
Raw materials
1of4
chevron_rightPreparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Nova Bioorganics Pvt Ltd | Andhra Pradesh, India | 13 | 58 | Inquiry | |
| Piramal Pharma Solutions | +919892735994 | Maharashtra, India | 48 | 58 | Inquiry |
| Aruvi Labs | +91-8056181939 +91-8056181939 | Tamil Nadu, India | 149 | 58 | Inquiry |
| PIRAMAL PHARMA LTD | +91-22-3802 3000 | New Delhi, India | 28 | 58 | Inquiry |
| TCI Chemicals (India) Pvt. Ltd. | 1800 425 7889 | New Delhi, India | 6778 | 58 | Inquiry |
| Pharmaffiliates Analytics and Synthetics P. Ltd | +91-172-5066494 | Haryana, India | 6773 | 58 | Inquiry |
| SynZeal Research Pvt Ltd | +1 226-802-2078 | Gujarat, India | 6522 | 58 | Inquiry |
| DR REDDYS LABORATORIES LTD | +91-40-49002900 | New Delhi, India | 201 | 58 | Inquiry |
| PIRAMAL ENTERPRISES LTD | +91-22-3802 3000/4000 | New Delhi, India | 45 | 58 | Inquiry |
| CLEARSYNTH LABS LTD. | +91-22-45045900 | Hyderabad, India | 6351 | 58 | Inquiry |





