Acetonitrile
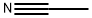
- CAS No.
- 75-05-8
- Chemical Name:
- Acetonitrile
- Synonyms
- ACN;MeCN;CH3CN;AN;Acetonitril;MGDA;anhydrous Acetonitrile;ETHANENITRILE;Cyanomethan;MOBILE PHASE ACETONITRILE
- CBNumber:
- CB2127174
- Molecular Formula:
- C2H3N
- Molecular Weight:
- 41.05
- MOL File:
- 75-05-8.mol
- MSDS File:
- SDS
- Modify Date:
- 2025/4/9 17:45:08
| Melting point | ?45 °C (lit.) |
|---|---|
| Boiling point | 81-82 °C (lit.) |
| Density | 0.786 g/mL at 25 °C (lit.) |
| vapor density | 1.41 (vs air) |
| vapor pressure | 72.8 mm Hg ( 20 °C) |
| refractive index |
n |
| Flash point | 48 °F |
| storage temp. | Store at +5°C to +30°C. |
| solubility | organic solvents: soluble(lit.) |
| pka | 25(at 25℃) |
| form | liquid |
| color | <10(APHA) |
| Specific Gravity | approximate 0.78(20/20℃) |
| Odor | Aromatic ether-like odor detectable at 40 ppm |
| Relative polarity | 0.46 |
| explosive limit | 3.0-17%(V) |
| Odor Threshold | 13ppm |
| Water Solubility | miscible |
| λmax |
λ: 195 nm Amax: ≤0.12 λ: 200 nm Amax: ≤0.032 λ: 230 nm Amax: ≤0.0044 λ: 235 nm Amax: ≤0.0044 λ: 250 nm Amax: ≤0.0044 λ: 400 nm Amax: ≤0.0044 |
| Merck | 14,70 |
| BRN | 741857 |
| Henry's Law Constant | 7.30 at 5 °C, 8.90 at 10 °C, 11.6 at 15 °C, 14.6 at 20 °C, 17.6 at 25 °C (headspace-GC, Ji and Evans, 2007) |
| Exposure limits | TLV-TWA 70 mg/m3 (40 ppm) (ACGIH and OSHA); STEL 105 mg/m3 (60 ppm) (ACGIH); IDLH 4000 ppm (NIOSH). |
| Dielectric constant | 37.5(21℃) |
| Stability | Incompatible with alkali metals, acids, bases, reducing agents and oxidizing agents. Highly flammable. |
| LogP | -0.340 |
| Surface tension | 28.95mN/m at 293.15K |
| CAS DataBase Reference | 75-05-8(CAS DataBase Reference) |
| NIST Chemistry Reference | Acetonitrile(75-05-8) |
| EPA Substance Registry System | Acetonitrile (75-05-8) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS02,GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H225-H302+H312+H332-H319 | |||||||||
| Precautionary statements | P210-P280-P301+P312-P303+P361+P353-P304+P340+P312-P305+P351+P338 | |||||||||
| Hazard Codes | F,Xi,Xn,T | |||||||||
| Risk Statements | 11-36-20/21/22-10-36/37/38-23/24/25-41-24-20/22 | |||||||||
| Safety Statements | 16-36/37-45-36/37/39-27-26-36 | |||||||||
| OEB | A | |||||||||
| OEL | TWA: 20 ppm (34 mg/m3) | |||||||||
| RIDADR | UN 1993 3/PG 3 | |||||||||
| WGK Germany | 2 | |||||||||
| RTECS | AL7700000 | |||||||||
| F | 9 | |||||||||
| Autoignition Temperature | 524 °C | |||||||||
| Hazard Note | Highly Flammable/Harmful/Irritant | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 3 | |||||||||
| PackingGroup | II | |||||||||
| HS Code | 29269095 | |||||||||
| Hazardous Substances Data | 75-05-8(Hazardous Substances Data) | |||||||||
| Toxicity | LD50 orally in rats: 3800 mg/kg (Smyth) | |||||||||
| IDLA | 137 ppm | |||||||||
| NFPA 704 |
|
Acetonitrile price More Price(102)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | RTC000080 | Acetonitrile Pharmaceutical Secondary Standard; Certified Reference Material | 75-05-8 | 20ML | ₹12351.33 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | PHR1551 | Acetonitrile Pharmaceutical Secondary Standard; Certified Reference Material | 75-05-8 | 3X1.2ML | ₹9103.83 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | PHR1320 | Residual Solvent - Acetonitrile Pharmaceutical Secondary Standard; Certified Reference Material | 75-05-8 | 3X1.2ML | ₹8270.3 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | L010000 | Acetonitrile | 75-05-8 | 6X100ML | ₹18911.28 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | L110000 | Acetonitrile | 75-05-8 | 45L | ₹162905.43 | 2022-06-14 | Buy |
Acetonitrile Chemical Properties,Uses,Production
Description
Acetonitrile is a liquid with an etherlike odor. It is a highly polar, volatile solvent used in many different industrial applications. It is widely used in the pharmaceutical, photographic, chemical, and analytical industries. It is useful as an industrial solvent for the separation of olefins, polymers, spinning fibers, and plastics. Other uses include the extraction and refining of copper and by-product ammonium sulfate; used for dyeing textiles and in coating compositions; used as a stabilizer for chlorinated solvents; manufacture of perfumes and cosmetics; and as a general reagent in a wide variety of chemical processes.
Chemical Properties
Acetonitrile (methyl cyanide), CH3CN, is a colorless liquid with a sweet, ethereal odor. It is completely miscible with water and its high dielectric strength and dipole moment make it an excellent solvent for both inorganic and organic compounds including polymers. it is commonly applied to the development and manufacturing of cosmetics, pharmaceutical and agricultural products.Acetonitrile has been banned in cosmetic products in the European Economic Area (EEA) since early 2000 and acetone and ethyl are often preferred as safer for domestic use.
Physical properties
Colorless liquid with an ether-like or pungent odor of vinegar. A detection odor threshold concentration of 1,950 mg/m3 (1,161 ppmv) was experimentally determined by Dravnieks (1974). An odor threshold concentration of 13 ppmv was reported by Nagata and Takeuchi (1990).
Uses
Acetonitrile is the simplest organic nitrile. It is a by-product of the manufacture of acrylonitrile, and acetonitrile has, in fact, replaced acrylonitrile. Acetonitrile has a number of uses, primarily as an extraction solvent for butadiene; as a chemical interme- diate in pesticide manufacturing; as a solvent for both inorganic and organic compounds; to remove tars, phenols, and coloring matter from petroleum hydrocarbons not soluble in acetonitrile; in the production of acrylic fi bers; in pharmaceuticals, perfumes, nitrile rubber, and acrylonitrile-butadiene-styrene (ABS) resins; in high-performance liquid and gas chro- matographic analysis; and in extraction and refi ning of copper. It is used as a starting material for the produc- tion of acetophenone, alpha-naphthalenacetic acid, thiamine, and acetamidine.
Production Methods
Acetonitrile is mainly prepared by dehydration of acetamide (CH3CONH2) with glacial acetic acid (Turner 1950) or by reacting acetic acid with ammonia at 400-500°C in the presence of a dehydration catalyst (Anon 1978).
Application
Acetonitrile is used as a solvent for polymers, spinning fibers, casting and molding plastics, and HPLC analyses; for extraction of butadiene and other olefins from hydrocarbon streams; in dyeing and coating textiles; and as a stabilizer for chlorinated solvents. It occurs in coal tar and forms as a by-product when acrylonitrile is made. Although acetonitrile is one of the more stable nitriles, it undergoes typical nitrile reactions and is used to produce many types of nitrogencontaining compounds.Acetonitrile also is used as a catalyst and as an ingredient in transitionmetal complex catalysts.
Definition
ChEBI: Acetonitrile is a nitrile that is hydrogen cyanide in which the hydrogen has been replaced by a methyl group. It has a role as a polar aprotic solvent and an EC 3.5.1.4 (amidase) inhibitor. It is an aliphatic nitrile and a volatile organic compound.
General Description
A colorless limpid liquid with an aromatic odor. Flash point 42°F. Density 0.783 c / cm3. Toxic by skin absorption. Less dense than water. Vapors are denser than air.
Air & Water Reactions
Highly flammable. Water soluble.
Reactivity Profile
Acetonitrile decomposes when heated to produce deadly toxic hydrogen cyanide gas and oxides of nitrogen. Strongly reactive [Hawley]. May react vigorously with strong oxidizing reagents, sulfuric acid, chlorosulfonic acid, sulfur trioxide, perchlorates, nitrating reagents, and nitric acid. [Sax, 9th ed., 1996, p. 20]. Potentially explosive in contact with nitrogen-fluorine compounds (e.g., tetrafluorourea) [Fraser, G. W. et al., Chem. Comm., 1966, p. 532].
Health Hazard
Acetonitrile liquid or vapor is irritating to the skin, eyes, and respiratory tract. Acetonitrile has only a modest toxicity, but it can be metabolized in the body to hydrogen cyanide and thiocyanate. Acetonitrile causes delayed symptoms of poisoning (several hours after the exposure) that include, but are not limited to, salivation, nausea, vomiting, anxiety, confusion, hyperpnea, dyspnea, respiratory distress, disturbed pulse rate, unconscious- ness, convulsions, and coma. Cases of acetonitrile poisoning in humans (or, more strictly, of cyanide poisoning after exposure to acetonitrile) are rare but not unknown, by inha- lation, ingestion, and (possibly) by skin absorption. Repeated exposure to acetonitrile may cause headache, anorexia, dizziness, weakness, and macular, papular, or vesicular dermatitis.
Flammability and Explosibility
Acetonitrile is a flammable liquid (NFPA rating = 3), and its vapor can travel a
considerable distance to an ignition source and "flash back." Acetonitrile vapor
forms explosive mixtures with air at concentrations of 4 to 16% (by volume).
Hazardous gases produced in a fire include hydrogen cyanide, carbon monoxide,
carbon dioxide, and oxides of nitrogen. Carbon dioxide or dry chemical
extinguishers should be used for acetonitrile fires.
Industrial uses
Acetonitrile is used as a solvent both in industry and in the laboratory, as a rodenticide, and in the denaturation of alcohol. Because of both its solvent properties and volatility, it is useful for extracting vegetable and animal oils and dissolving hydrocarbons, oils, and greases. Acetonitrile is used for the purification of acetylene and artificial textile fibers, and as an antioxidant for rubber (Dequidt et al 1974). It has also been used to extract herbicide residues from soils (Smith 1980), to remove tars and other compounds from petroleum hydrocarbons, and to extract fatty acids from vegetable and fish liver oil. Acetonitrile is now a standard solvent component in reversed-phase high-performance liquid chromatography. It is the starting point for the syntheses of a number of organic compounds such as carboxylic acids and various nitrogen derivatives (Smiley 1981).
Safety Profile
Poison by ingestion and intraperitoneal routes. Moderately toxic by several routes. An experimental teratogen. Other experimental reproductive effects. A skin and severe eye irritant. Human systemic effects by ingestion: convulsions, nausea or vomiting, and metabolic acidosis. Human respiratory system effects by inhalation. Mutation data reported. Dangerous fire hazard when exposed to heat, flame, or oxidizers. Explosion Hazard: See also CYANIDE and NITRILES. When heated to decomposition it emits highly toxic fumes of CNand NOx,. Potentially explosive reaction with lanthanide perchlorates and nitrogen-fluorine compounds. Exothermic reaction with sulfuric acid at 53°C. Will react with water, steam, acids to produce toxic and flammable vapors. Incompatible with oleum, chlorosulfonic acid, perchlorates, nitrating agents, inchum, dinitrogen tetraoxide, N-fluoro compounds (e.g., perfluorourea + acetonitrile), HNO3, so3. To fight fire, use foam, Con, dry chemical
Potential Exposure
Acetonitrile is used as an extractant for animal and vegetable oils, as a solvent; particularly in the pharmaceutical industry, and as a chemical intermediate in pesticide manufacture; making batteries and rubber products. It is present in cigarette smoke
Carcinogenicity
Under the conditions of these 2- year inhalation studies by NTP, there was equivocal evidence of carcinogenic activity of acetonitrile in male F344/N rats based on marginally increased incidences of hepatocellular adenoma and carcinoma. There was no evidence of carcinogenic activity of acetonitrile in female F344/N rats exposed to 100, 200, or 400 ppm. There was no evidence of carcinogenic activity of acetonitrile in male or female B6C3F1 mice exposed to 50, 100, or 200 ppm. Exposure to acetonitrile by inhalation resulted in increased incidences of hepatic basophilic foci in male rats and of squamous hyperplasia of the forestomach in male and female mice.
Metabolism
Acetonitrile metabolism in dogs was demonstrated by Lang (1894), who reported that about 20% of the nitrile administered was converted to thio-cyanate in the urine, while guinea pigs metabolized acetonitrile to a greater extent (50% of dose excreted as thiocyanate). When the animals were pre-treated with ethanol, acetonitrile metabolism was induced (Tanii and Hashimoto 1986). In rats, acetone was found to potentiate acetonitrile toxicity and elevate cyanide concentrations in the blood (Freeman and Hays 1985). Baumann et al (1933) found that rabbits injected with acetonitrile excreted 27-35% of the dose as thiocyanate, while in thyroidectomized rabbits, the excretion decreased significantly (3-5% of the dose). Thiocyanate excretion was increased notably upon feeding dessicated thyroid to these animals. Hunt (1923) found that powdered sheep thyroid protected mice against acetonitrile toxicity. However, the role played by the thyroid in the detoxication of cyanide to thiocyanate is unclear. It has been suggested that the thyroid may have a role in the microsomal cleavage of cyanide from acetonitrile other than its direct effect on sulphation of cyanide to thiocyanate.
storage
Acetonitrile should be used only in areas free of ignition sources, and quantities greater than 1 liter should be stored in tightly sealed metal containers in areas separate from oxidizers.
Shipping
UN1648 Acetonitrile, Hazard Class: 3; Labels: 3-Flammable liquid
Incompatibilities
Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, chlorosulfonic acid, oleum, epoxides. May accumulate static electrical charges, and may cause ignition of its vapors. Nitriles may polymerize in the presence of metals and some metal compounds. They are incompatible with acids; mixing nitriles with strong oxidizing acids can lead to extremely violent reactions. Nitriles are generally incompatible with other oxidizing agents such as peroxides and epoxides. The combination of bases and nitriles can produce hydrogen cyanide. Nitriles are hydrolyzed in both aqueous acid and base to give carboxylic acids (or salts of carboxylic acids). These reactions generate heat. Peroxides convert nitriles to amides. Nitriles can react vigorously with reducing agents. Acetonitrile and propionitrile are soluble in water, but nitriles higher than propionitrile have low aqueous solubility. They are also insoluble in aqueous acids
Waste Disposal
Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal. Incineration with nitrogen oxide removal from effluent gases by scrubbers or incinerators
Acetonitrile Preparation Products And Raw materials
Raw materials
1of3
chevron_rightPreparation Products
1of8
chevron_right| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| ANJI BIOSCIENCES | +91-9000100077 +91-9000100077 | Hyderabad, India | 429 | 58 | Inquiry |
| Leonid Chemicals Pvt Ltd | +918023378354 | Karnataka, India | 16 | 58 | Inquiry |
| Soham Chemical Industries | +91-7016081644 +91-7016081644 | Mumbai, India | 82 | 58 | Inquiry |
| oswal chemicals | +91-9173644055 +91-9173644055 | Gujarat, India | 66 | 58 | Inquiry |
| Merck Ltd | +91-2262109800 +91-2262109000 | Maharashtra, India | 272 | 58 | Inquiry |
| Sahastra Chemicals Private Limited | +91-9011434239 +91-9011434239 | Maharashtra, India | 3 | 58 | Inquiry |
| Deepak Novochem Technologies Ltd | +91-2066090244 +91-8888854344 | Mumbai, India | 17 | 58 | Inquiry |
| Kairav Chemofarbe Industries Ltd. KCIL | +91-222596836 +91-8879003729 | Maharashtra, India | 9 | 58 | Inquiry |
| Sujay Chemicals | +91-2227700303 +91-9323420477 | Maharashtra, India | 7 | 58 | Inquiry |
| DeFINE CHEMICALS | +91-8010769889 +91-9320057099 | Maharashtra, India | 120 | 58 | Inquiry |
| Supplier | Advantage |
|---|---|
| ANJI BIOSCIENCES | 58 |
| Leonid Chemicals Pvt Ltd | 58 |
| Soham Chemical Industries | 58 |
| oswal chemicals | 58 |
| Merck Ltd | 58 |
| Sahastra Chemicals Private Limited | 58 |
| Deepak Novochem Technologies Ltd | 58 |
| Kairav Chemofarbe Industries Ltd. KCIL | 58 |
| Sujay Chemicals | 58 |
| DeFINE CHEMICALS | 58 |
Related articles
- Is acetonitrile a highly polar solvent?
- Acetonitrile is a highly polar, volatile organic compound used as a solvent in many industrial applications.
- Dec 27,2023
- Effect of Additives on Acetonitrile Polarity
- Acetonitrile is a relatively nontoxic, highly volatile and aprotic polar organic solvent, some additives can affect its polari....
- Dec 21,2023
- What is Acetonitrile?
- Acetonitrile is a liquid with an etherlike odor. It is a highly polar, volatile solvent used in many different industrial appl....
- Oct 14,2021
75-05-8(Acetonitrile )Related Search:
1of4
chevron_right




