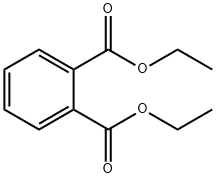
Diethyl phthalate
- CAS No.
- 84-66-2
- Chemical Name:
- Diethyl phthalate
- Synonyms
- Diethyl ester;ETHYL PHTHALATE;Diethy phthalate;PHTHALOL;TETRATHIONATE;Diethyl phthlate;Diethyl-o-phthalate;PHTHALIC ACID, BIS-ETHYL ESTER;Anozol;DIETHYLPHTALATE
- CBNumber:
- CB3349654
- Molecular Formula:
- C12H14O4
- Molecular Weight:
- 222.24
- MOL File:
- 84-66-2.mol
- MSDS File:
- SDS
- Modify Date:
- 2024/6/19 22:55:17
| Melting point | -3 °C (lit.) |
|---|---|
| Boiling point | 298-299 °C (lit.) |
| Density | 1.12 g/mL at 25 °C (lit.) |
| vapor density | 7.66 (vs air) |
| vapor pressure | 1 mm Hg ( 100 °C) |
| refractive index |
n |
| Flash point | >230 °F |
| storage temp. | 2-8°C |
| solubility | Practically insoluble in water, miscible with ethanol (96 per cent). |
| form | Liquid |
| color | APHA: ≤15 |
| Specific Gravity | 1.118 |
| Odor | water-wh. oily liq., odorless, bitter taste |
| explosive limit | 0.75%, 187°F |
| Water Solubility | 1 g/L (20 ºC) |
| Merck | 14,7371 |
| BRN | 1912500 |
| Henry's Law Constant | At 25 °C: 5.01, 4.54, 4.78, 4.94, 2.21, and 2.44 (x 10-5 atm?m3/mol)at pH values of 2.96, 2.98, 6.18, 6.19, 8.98, and 9.00, respectively (Hakuta et al., 1977). |
| Exposure limits | TLV-TWA air 5 mg/m3 (ACGIH). . |
| Dielectric constant | 7.1(45℃) |
| Stability | Stable. Combustible. Incompatible with strong oxidizing agents, strong acids, alkalies. |
| LogP | 2.2 at 41℃ |
| CAS DataBase Reference | 84-66-2(CAS DataBase Reference) |
| NIST Chemistry Reference | Diethyl phthalate(84-66-2) |
| EPA Substance Registry System | Diethyl phthalate (84-66-2) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS08 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H361 | |||||||||
| Precautionary statements | P201-P202-P280-P308+P313-P405-P501a | |||||||||
| Safety Statements | 24/25 | |||||||||
| RIDADR | UN 3082 9 / PGIII | |||||||||
| OEB | B | |||||||||
| OEL | TWA: 5 mg/m3 | |||||||||
| WGK Germany | 2 | |||||||||
| RTECS | TI1050000 | |||||||||
| Autoignition Temperature | 854 °F | |||||||||
| TSCA | Yes | |||||||||
| HS Code | 29173400 | |||||||||
| Toxicity | LD50 i.p. in rats: 5.06 ml/kg (Singh) | |||||||||
| NFPA 704 |
|
Diethyl phthalate price More Price(18)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | W512206 | Diethyl phthalate ≥99% | 84-66-2 | 1SAMPLE | ₹5141.88 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | W512206 | Diethyl phthalate ≥99% | 84-66-2 | 1KG | ₹6852.23 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | W512206 | Diethyl phthalate ≥99% | 84-66-2 | 25KG | ₹31316.73 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 524972 | Diethyl phthalate 99.5% | 84-66-2 | 5ML | ₹3312.45 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 524972 | Diethyl phthalate 99.5% | 84-66-2 | 100ML | ₹3680.5 | 2022-06-14 | Buy |
Diethyl phthalate Chemical Properties,Uses,Production
Description
Diethyl phthalate (DEP) is a member of the group of esters of phthalic acid known as phthalates, used ubiquitously as solvents and plasticisers worldwide. DEP can increases the flexibility of plastics. It is also contained in deodorant formulations, perfumes, emollients and insect repellents. It can cross react with dimethyl phtalate.
Chemical Properties
Diethyl phthalate is a clear, colorless, oily liquid. It is practically odorless, or with a very slight aromatic odor and a bitter, disagreeable taste. It is miscible with ethanol and ethyl ether. Diethyl phthalate is soluble in acetone, benzene, carbon tetrachloride, alcohols, ketones, esters, and aromatic hydrocarbons and partly miscible with aliphatic solvents. Diethyl phthalate, when exposed to heat, decomposes and emits acrid smoke and irritating fumes. It is incompatible with strong oxidisers, strong acids, nitric acid, permanganates, and water and attacks some forms of plastics. Diethyl phthalate is produced in the reaction of phthalic anhydride with ethanol in the presence of concentrated sulphuric acid catalyst.
Physical properties
Clear, colorless, oily liquid with a mild, chemical odor. Bitter taste.
Virtually odorless when pure ("Perfumery
grade" of commercial Diethyl phthalate).
Very bitter taste in weak hydro-alcoholic
solution. Bitter taste perceptible below 20 ppm.
Uses
Diethyl phthalate is a Plasticizer for cellulose ester plastic films and sheets; in molded plastics; manufacturing varnishes; cosmetics.
Production Methods
Diethyl phthalate is produced by the reaction of phthalic anhydride with ethanol in the presence of sulfuric acid.
Definition
ChEBI: The diethyl ester of benzene-1,2-dicarboxylic acid.
General Description
A clear, colorless liquid without significant odor. More dense than water and insoluble in water. Hence sinks in water. Primary hazard is to the environment. Spread to the environment should be immediately stopped. Easily penetrates soil, contaminates groundwater and nearby waterways. Flash point 325°F. Severely irritates eyes and mildly irritates skin. Used in the manufacture of perfumes, plastics, mosquito repellents and many other products.
Air & Water Reactions
Insoluble in water.
Reactivity Profile
Diethyl phthalate is an ester. Esters react with acids to liberate heat along with alcohols and acids. Strong oxidizing acids may cause a vigorous reaction that is sufficiently exothermic to ignite the reaction products. Heat is also generated by the interaction of esters with caustic solutions. Flammable hydrogen is generated by mixing esters with alkali metals and hydrides. Can generate electrostatic charges. [Handling Chemicals Safely 1980. p. 250].
Health Hazard
Diethyl phthalate exhibited low to very lowacute toxicity in laboratory animals. Inges tion produced somnolence and hypotension.Inhalation of its vapors may result in lacrima tion, coughing, and irritation of the throatin humans. The oral LD50 value in mice is6170 mg/kg. Diethyl phthalate administeredto pregnant rats at 5% concentration in thefeed showed no adverse effect upon embryoor fetal growth, viability, or the incidence ofmalformations (Price et al. 1988).
Fire Hazard
Special Hazards of Combustion Products: Irritating vapors of unburned chemical may form in fire.
Pharmaceutical Applications
Diethyl phthalate is used as a plasticizer for film coatings on tablets, beads, and granules at concentrations of 10–30% by weight of polymer.
Diethyl phthalate is also used as an alcohol denaturant and as a solvent for cellulose acetate in the manufacture of varnishes and dopes. In perfumery, diethyl phthalate is used as a perfume fixative at a concentration of 0.1–0.5% of the weight of the perfume used.
Contact allergens
This plasticizer increases the fexibility of plastics. It is also contained in deodorant formulations, perfumes, emollients, and insect repellents. It can cross-react with dimethyl phthalate.
Safety Profile
Poison by intravenous route. Moderately toxic by ingestion, subcutaneous, and intraperitoneal routes. Human systemic effects by inhalation: lachrymation, respiratory obstruction, and other unspecified respiratory system effects. An eye irritant and systemic irritant by inhalation. An experimental teratogen. Other experimental reproductive effects. Narcotic in hgh concentrations. Combustible when exposed to heat or flame. To fight fire, use water spray, mist, foam. When heated to decomposition it emits acrid smoke and irritating fumes.
Safety
Diethyl phthalate is used in oral pharmaceutical formulations and is generally regarded as a nontoxic and nonirritant material at the levels employed as an excipient. However, if consumed in large quantities it can act as a narcotic and cause paralysis of the central nervous system.
Although some animal studies have suggested that high concentrations of diethyl phthalate may be teratogenic, other studies have shown no adverse effects.
LD50 (guinea pig, oral): 8.6g/kg
LD50 (mouse, IP): 2.7g/kg
LD50 (mouse, oral): 6.2g/kg
LD50 (rat, IP): 5.1g/kg
LD50 (rat, oral): 8.6g/kg
Environmental Fate
Biological. A proposed microbial degradation mechanism is as follows: 4-hydroxy-3-
methylbenzyl alcohol to 4-hydroxy-3-methylbenzaldehyde to 3-methyl-4-hydroxybenzoic acid to
4-hydroxyisophthalic acid to protocatechuic acid to β-ketoadipic acid (Chapman, 1972). In
anaerobic sludge, diethyl phthalate degraded as follows: monoethyl phthalate to phthalic acid to
protocatechuic acid followed by ring cleavage and mineralization (Shelton et al., 1984).
Photolytic. An aqueous solution containing titanium dioxide and subjected to UV radiation (λ
>290 nm) produced hydroxyphthalates and dihydroxyphthalates as intermediates (Hustert and
Moza, 1988).
Chemical/Physical. Under alkaline conditions, diethyl phthalate will initially hydrolyze to ethyl
hydrogen phthalate and ethanol. The monoester will undergo hydrolysis forming o-phthalic acid
and ethanol (Kollig, 1993). A second-order rate constant of 2.5 x 10-2/M?sec was reported for the
hydrolysis of diethyl phthalate at 30 °C and pH 8 (Wolfe et al., 1980). At 30 °C, hydrolysis halflives
of 8.8 and 18 yr were reported at pH values 9 and 10-12, respectively (Callahan et al., 1979).
storage
Diethyl phthalate is stable when stored in a well-closed container in a cool, dry place.
Purification Methods
Wash the ester with aqueous Na2CO3, then distilled water, dry (CaCl2), and distil it under reduced pressure. Store it in a vacuum desiccator over P2O5. [Beilstein 9 IV 3172.]
Incompatibilities
Incompatible with strong oxidizing materials, acids, and permanganates.
Regulatory Status
Included in the FDA Inactive Ingredients Database (oral capsules, delayed action, enteric coated, and sustained action tablets). Included in nonparenteral medicines licensed in the UK. Included in the Canadian List of Acceptable Non-medicinal Ingredients.
Diethyl phthalate Preparation Products And Raw materials
Raw materials
Preparation Products
1of3
chevron_right| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| ADVENT CHEMBIO PRIVATE LIMITED | +91-9821524116 +91-9821524116 | Mumbai, India | 353 | 58 | Inquiry |
| Rsum Industries Pvt. Ltd. | +91-91062685255 +91-91062685255 | New Delhi, India | 29 | 58 | Inquiry |
| Soham Chemical Industries | +91-7016081644 +91-7016081644 | Mumbai, India | 83 | 58 | Inquiry |
| SHIVAM INDUSTRIES | +91-9820823043 +91-9820823043 | Mumbai, India | 30 | 58 | Inquiry |
| HJ AROCHEM | +91-8690768060 +91-9724420086 | Ahmedabad, India | 171 | 58 | Inquiry |
| Ultra Chemical Works | +91-9820078105 +91-9820078105 | Mumbai, India | 124 | 58 | Inquiry |
| KLJ Group | +91-1141427427 +91-1145371400 | New Delhi, India | 29 | 58 | Inquiry |
| Indenta Chemicals (India) Pvt Ltd | +91-2226849600 +91-9326627752 | Maharashtra, India | 48 | 58 | Inquiry |
| Indo Nippon Chemical Co Ltd | +91-2222051723 +91-2222051723 | Maharashtra, India | 1 | 58 | Inquiry |
| Supreme Plasticizers | +91-9845114149 +91-9845170909 | Maharashtra, India | 3 | 58 | Inquiry |
| Supplier | Advantage |
|---|---|
| ADVENT CHEMBIO PRIVATE LIMITED | 58 |
| Rsum Industries Pvt. Ltd. | 58 |
| Soham Chemical Industries | 58 |
| SHIVAM INDUSTRIES | 58 |
| HJ AROCHEM | 58 |
| Ultra Chemical Works | 58 |
| KLJ Group | 58 |
| Indenta Chemicals (India) Pvt Ltd | 58 |
| Indo Nippon Chemical Co Ltd | 58 |
| Supreme Plasticizers | 58 |
Related articles
- Application of diethyl phthalate in production and life
- Diethyl phthalate is a chemical with the molecular formula C12H14O4.
- Apr 8,2022





