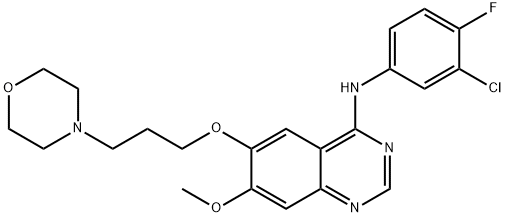
Gefitinib
- CAS No.
- 184475-35-2
- Chemical Name:
- Gefitinib
- Synonyms
- IRESSA;Gefinitib;Gifitinib;Gefitinib Tablets;N-(3-Chloro-4-fluorophenyl)-7-methoxy-6-[3-(4-morpholinyl)propoxy]-4-quinazolinamine;N-(3-Chlor-4-fluorophenyl)-7-[methoxy-6-[(3-morpholin-4-yl)propoxyl]-quinazolin-4-yl]amine;CS-524;ZD 1839;efitinib;GEFITINIB
- CBNumber:
- CB8120056
- Molecular Formula:
- C22H24ClFN4O3
- Molecular Weight:
- 446.9
- MOL File:
- 184475-35-2.mol
- MSDS File:
- SDS
- Modify Date:
- 2024/8/21 22:41:43
| Melting point | 119-1200C |
|---|---|
| Boiling point | 586.8±50.0 °C(Predicted) |
| Density | 1.322±0.06 g/cm3(Predicted) |
| storage temp. | room temp |
| solubility | Soluble in DMSO (up to 40 mg/ml) or in Ethanol (up to 4 mg/ml). |
| pka | 7.00±0.10(Predicted) |
| form | powder |
| color | white to beige |
| Stability | Stable for 2 years from date of purchase as supplied. Solutions in DMSO or ethanol may be stored at -20°C for up to 1 month. |
| CAS DataBase Reference | 184475-35-2(CAS DataBase Reference) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H302-H315-H319-H335 | |||||||||
| Precautionary statements | P301+P312+P330-P305+P351+P338 | |||||||||
| Safety Statements | 24/25 | |||||||||
| HS Code | 29349990 | |||||||||
| NFPA 704 |
|
Gefitinib price More Price(4)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | SML1657 | Gefitinib ≥98% (HPLC) | 184475-35-2 | 10MG | ₹11355.43 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | SML1657 | Gefitinib ≥98% (HPLC) | 184475-35-2 | 50MG | ₹45778.93 | 2022-06-14 | Buy |
| TCI Chemicals (India) | G0546 | Gefitinib | 184475-35-2 | 1G | ₹4200 | 2022-05-26 | Buy |
| TCI Chemicals (India) | G0546 | Gefitinib | 184475-35-2 | 5G | ₹11200 | 2022-05-26 | Buy |
Gefitinib Chemical Properties,Uses,Production
Description
Gefitinib was introduced in Japan as a daily oral monotherapy for the treatment of inoperable or recurrent non-small cell lung cancers (NSCLC). This anilinoquinazoline derivative can be synthesized in 6 steps starting from 6,7-dimethoxyquinazolin-4(3H)-one by successive monodemethylationlacetylation of the 6-hydroxy-group followed by chlorination and reaction with 3-chloro-4-fluoroaniline, finally deacetylation and alkylation with 3-(4-morpholinyl)propylbromide complete the synthesis. Gefitinib reversibly inhibits the activity of the epidermal growth factor receptor tyrosine kinase (EGRF TK). This inhibits autophosphorylation of EGRF and blocks the cascade of intracellular events which have been implicated in the proliferation, survival and metastasis of cancer cells. Gefitinib diplays good selectivity for the EGRF TK relative to other growth factors in human umbilical endothelial cells. It is similarly selective relative to other kinases, for example cerB2. Data from two large phase II studies in patients with pretreated NSCLC have shown that gefitinib induces a response rate approaching 20% in patients receiving the agent as a second line therapy and approximately 10% in those pretreated with more lines of chemotherapy. Gefitinib has good bioavailability and is metabolized in the liver via the cytochrome P450 3A4 enzyme system with a mean elimination half life of 28 h. Gefitinib has been generally well tolerated in cancer patients with predominant side effects being acne-like skin-rash, diarrhea, nausea, vomiting and mild to moderate myelosuppression. .
Chemical Properties
Light-Yellow Crystalline Powder
Uses
Gefitinib is an antineoplastic.
Indications
The EGFR or ErbB1 inhibitor gefitinib (Iressa(R), AstraZeneca) was originally approved by the US FDA in 2003 under accelerated regulations for the treatment of locally advanced or metastatic non-small cell lung cancer (NSCLC) after progression on docetaxel- and platinum-based chemotherapy. AstraZeneca voluntarily withdrew gefitinib from the market in 2005, owing to failed verification of clinical benefit during post-approval studies. In July 2015, FDA reinstated the approval of gefitinib for a different group of patients (i.e., NSCLC patients with EGFR mutations).
Other approved kinase inhibitors targeting the ErbB family, which includes ErbB1/EGFR, ErbB2/human epidermal growth factor receptor 2 (Her2), ErbB3/ Her3, and ErbB4/Her4, are erlotinib (Tarceva(R), OSI Pharm.), lapatinib (Tykerb(R), GlaxoSmithKline), vandetanib (Caprelsa(R), AstraZeneca), afatinib (Gilotrif(R), Boehringer Ingelheim) , and osimertinib (Tagrisso(R), AstraZeneca). All approved EGFR family inhibitors share a common quinazoline scaffold with the exception of osimertinib, which has a pyrimidinylphenylamine scaffold that resembles that of imatinib and nilotinib. Gefitinib and vandetanib adopt the type I binding mode with “DFG-in” and αC-helix “in” conformation, while erlotinib and lapatinib bind to“DFG-in”with the αC-helix adopting an “out” conformation. Afatinib and osimertinib are covalent inhibitors with an electrophilic enone moiety.
General Description
Geftinib is available as 250-mg tablets for oral administrationin the treatment of NSCLC for those patients who have failedto respond to platinum-based therapies and docetaxel and hasalso been used against squamous cell cancers of the head andneck. The agent is an inhibitor of the TK of EGF-R and possiblyother TKs as well. Gefitinib is both a substrate and inhibitorof Pgp and BCRP. The agent is absorbed slowly afterbeing administered orally with 60% bioavailability.Metabolism occurs in the liver and is mediated primarily byCYP3A4 to give eight identified metabolites resulting fromdefluorination of the phenyl ring, oxidative-O-demethylation,and multiple products arising as a result of oxidation of themorpholine ring. The O-demethylated product represents thepredominate metabolite and is 14-fold less active comparedwith the parent. The parent and metabolites are eliminated inthe feces with a terminal elimination half-life of 48 hours.The drug appears to be well tolerated with the most commonlyreported side effects being rash and diarrhea. It mayalso cause elevations in blood pressure especially in those patientswith preexisting hypertension, elevation of transaminaselevels, and mild nausea and mucositits.
General Description
Class: receptor tyrosine kinase
Treatment: NSCLC
Oral bioavailability = 60%
Elimination half-life = 48 h
Protein binding = 97%
Biological Activity
Orally active, selective inhibitor of EGFR tyrosine kinase (IC 50 = 23-79 nM). Shows minimal activity against ErbB2, KDR, c-flt, PKC, MEK and ERK-2. Blocks EGFR autophosphorylation and inhibits tumor growth in mice bearing a range of human xenografts.
Gefitinib Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| SUJALAM CHEMICALS | +91-9422560788 +91-9422560788 | Maharashtra, India | 42 | 58 | Inquiry |
| CDYMAX | +91-9019317788 +91-9019317788 | Bangalore, India | 28 | 58 | Inquiry |
| Gonane Pharma | +91-9819380043 +91-9819380043 | NaviMumbai, India | 192 | 58 | Inquiry |
| Hetero Drugs Limited | +91-4023704923 +91-4023704923 | Telangana, India | 296 | 58 | Inquiry |
| Zyphars Biopharmaceuticals Pvt. Ltd | +91-8007587007 +91-8007587007 | Mumbai, India | 14 | 58 | Inquiry |
| Basil Drugs AND Pharmaceuticals Pvt Ltd | +91-2249700250 +91-9619320820 | Mumbai, India | 108 | 58 | Inquiry |
| NATCO Pharma Limited | +91-9849511345 +91-9866005199 | Hyderabad, India | 24 | 58 | Inquiry |
| Humble Healthcare Limited | +91-9720093000 +91-8006400378 | Uttar Pradesh, India | 141 | 58 | Inquiry |
| Kekule Pharma Limited | +919849798889 | Hyderabad, India | 59 | 58 | Inquiry |
| Raising Sun Pharma | +91-9399941155 +91-9399941155 | Telangana, India | 127 | 58 | Inquiry |
| Supplier | Advantage |
|---|---|
| SUJALAM CHEMICALS | 58 |
| CDYMAX | 58 |
| Gonane Pharma | 58 |
| Hetero Drugs Limited | 58 |
| Zyphars Biopharmaceuticals Pvt. Ltd | 58 |
| Basil Drugs AND Pharmaceuticals Pvt Ltd | 58 |
| NATCO Pharma Limited | 58 |
| Humble Healthcare Limited | 58 |
| Kekule Pharma Limited | 58 |
| Raising Sun Pharma | 58 |
Related articles
- Gefitinib vs Erlotinib vs Afatinib: differences in anticancer drugs
- Gefitinib, erlotinib and afatinib are all anticancer drugs widely used in the treatment of advanced non-small cell lung cancer....
- Mar 28,2024
- Gefitinib: Pharmacodynamic Properties, Dosage and Administration
- Gefitinib demonstrates pharmacodynamic properties in inhibiting downstream signal transduction in NSCLC, and its recommended d....
- Jan 23,2024
- Gefitinib: mechanism of action, pharmacokinetics and side effect
- Gefitinib targets EGFR to inhibit cancer cell growth, particularly in NSCLC with specific mutations, and has a favorable toxic....
- Sep 28,2023
184475-35-2(Gefitinib)Related Search:
1of4
chevron_right




