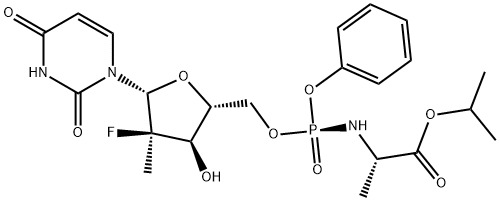
Sofosbuvir
- CAS No.
- 1190307-88-0
- Chemical Name:
- Sofosbuvir
- Synonyms
- Suofeibuwei;(S)-isopropyl 2-(((S)-(((2R,3R,4R,5R)-5-(2,4-dioxo-3,4-dihydropyriMidin-1(2H)-yl)-4-fluoro-3-hydroxy-4-Methyltetrahydrofuran-2-yl)Methoxy)(phenoxy)phosphoryl)aMino)propanoate;Sophy Bouvet;propan-2-yl (2S)-2-[[[(2R,3R,4R,5R)-5-(2,4-dioxopyrimidin-1-yl)-4-fluoro-3-hydroxy-4-methyloxolan-2-yl]methoxy-phenoxyphosphoryl]amino]propanoate;propan-2-yl (2S)-2-[[[(2R,3R,4R,5S)-5-(2,4-dioxopyrimidin-1-yl)-4-fluoro-3-hydroxy-4-methyloxolan-2-yl]methoxy-phenoxyphosphoryl]amino]propanoate;Isopropyl (2S)-2-{[(S)-{[(2R,3R,4R,5R)-5-(2,4-dioxo-3,4-dihydro-1(2H)-pyrimidinyl)-4-fluoro-3-hydroxy-4-methyltetrahydro-2-furanyl]methoxy}(phenoxy)phosphoryl]amino}propanoate;GS-7977;PSI 7977;Sofosbubir;sofosbuvir
- CBNumber:
- CB02604350
- Molecular Formula:
- C22H29FN3O9P
- Molecular Weight:
- 529.45
- MOL File:
- 1190307-88-0.mol
- Modify Date:
- 2025/4/29 15:35:11
| Melting point | 122-124°C |
|---|---|
| Density | 1.41 |
| storage temp. | Hygroscopic, -20°C Freezer, Under inert atmosphere |
| solubility | DMSO (Slightly), Methanol (Slightly) |
| form | Solid |
| pka | 9.39±0.10(Predicted) |
| color | White |
| InChIKey | TTZHDVOVKQGIBA-YBSJRAAASA-N |
| SMILES | C(OC(C)C)(=O)[C@@H](N[P@@](OC[C@@H]1[C@@H](O)[C@](F)(C)[C@H](N2C(=O)NC(=O)C=C2)O1)(OC1=CC=CC=C1)=O)C |
| CAS DataBase Reference | 1190307-88-0 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS07,GHS08 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H373 | |||||||||
| Precautionary statements | P260-P314-P501 | |||||||||
| HS Code | 29339900 | |||||||||
| Hazardous Substances Data | 1190307-88-0(Hazardous Substances Data) | |||||||||
| NFPA 704 |
|
Sofosbuvir Chemical Properties,Uses,Production
Description
Sofosbuvir is a drug used for the treatment of hepatitis C. It is recommended to be used in combination with other drugs (such as velpatasvir) for the first-line treatment for HCV genotypes 1, 2, 3, 4, 5, and 6. It takes effect through acting as a nucleotide analog inhibitor, being capable of specially inhibiting the HCV NS5B (non-structural protein 5B) RNA-dependent RNA polymerase.
Uses
PSI-7977 is a prodrug that is metabolized to the active antiviral agent 2'-deoxy-2'-α-fluoro-β-C-methyluridine-5'-monophosphate and is currently being investigated in phase 3 clinical trials for the t reatment of hepatitis C. Studies have profiled PSI-7977 as a nucleotide inhibitor of hepatitis C virus, exerting selective inhibitory effects towards HCV NS5B polymerase.
Definition
ChEBI: A nucleotide conjugate that is used in combination with ledipasvir (under the trade name Harvoni) for the treatment of chronic hepatitis C genotype 1 infection.
Clinical Use
In December 2013, sofosbuvir (also known as GS-7977 and PSI-7977) was approved in the United States for the treatment of hepatitis C virus (HCV) infection as a component of a combination antiviral treatment regimen. Sofosbuvir was discovered from an effort to enhance the activity of the parent nucleoside by bypassing rate-limiting monophosphorylation with a prodrug that would liberate the intactmonophosphate in the liver, where it would then be converted by cellular kinases to the active triphosphate species. In addition, the prodrug was designed to be amenable to oral delivery. In the initial synthesis of sofosbuvir, the iso-propyl ester of (L)-alanine was coupled with phenyl dichlorophosphate to provide a diastereomeric intermediate that was coupled with the uridine nucleoside. The diastereomeric mixture (GS-9851) was shown to produce high levels of triphosphate in vitro in primary hepatocytes and in the livers of rats, dogs, and monkeys after oral dosing. The individual diastereomers were obtained by chromatography or by crystallization. The diastereomer with the Sp configuration at the phosphorous center (sofosbuvir) was found to be >10-fold more potent in an HCV replicon assay than the corresponding Rp diastereomer (EC90s of 0.42 and 7.5 μM, respectively).
Sofosbuvir Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Jigs Chemical ltd | +919099003427 | Gujarat, India | 239 | 58 | Inquiry |
| Bulat Pharmaceutical Pvt Ltd | +91-8448085659 +91-8448085660 | Haryana, India | 123 | 58 | Inquiry |
| J S LABS | +91-7330612784 +91-7330612784 | Tamil Nadu, India | 159 | 58 | Inquiry |
| Viruj Pharmaceuticals Pvt Ltd | +91-9441591589 +91-9441591589 | Hyderabad, India | 33 | 58 | Inquiry |
| Varanous Labs Pvt Ltd | +91-7036248882 +91-7036248882 | Hyderabad, India | 1537 | 58 | Inquiry |
| Prayosha Health Care Pvt Ltd | +91-9924448881 +91-9924448881 | Gujarat, India | 15 | 58 | Inquiry |
| Lupin Ltd | +91-8019896181 +91-8019896181 | Maharashtra, India | 93 | 58 | Inquiry |
| Alembic Pharmaceuticals Limited | +91-2652280880 +91-2652280550 | Gujarat, India | 115 | 58 | Inquiry |
| Hetero Drugs Limited | +91-4023704923 +91-4023704923 | Telangana, India | 296 | 58 | Inquiry |
| VIVIN DRUGS AND PHARMACEUTICALS LTD | +91-22670456625 +91-224067045635 | Telangana, India | 22 | 58 | Inquiry |
| Supplier | Advantage |
|---|---|
| Jigs Chemical ltd | 58 |
| Bulat Pharmaceutical Pvt Ltd | 58 |
| J S LABS | 58 |
| Viruj Pharmaceuticals Pvt Ltd | 58 |
| Varanous Labs Pvt Ltd | 58 |
| Prayosha Health Care Pvt Ltd | 58 |
| Lupin Ltd | 58 |
| Alembic Pharmaceuticals Limited | 58 |
| Hetero Drugs Limited | 58 |
| VIVIN DRUGS AND PHARMACEUTICALS LTD | 58 |
Related articles
- Sofosbuvir: Characteristics and Pharmacokinetics
- Sofosbuvir is an effective, orally administered antiviral for hepatitis C with high absorption, favorable pharmacokinetics, an....
- Nov 22,2024
- Sofosbuvir: Indications, Mechanism of Action and Side Effects
- Sofosbuvir is a direct-acting antiviral drug primarily used in combination with other drugs to treat hepatitis C virus (HCV) i....
- Aug 16,2024
- The review of sofosbuvir
- Sofosbuvir, a prodrug metabolized to the active antiviral drug 2'-deoxy-2'-α-fluoro-β-C-methyluridine-5'-monophosphate, is cur....
- Feb 23,2022
1190307-88-0(Sofosbuvir)Related Search:
1of4
chevron_right




