CYANOGEN IODIDE
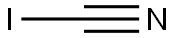
- CAS No.
- 506-78-5
- Chemical Name:
- CYANOGEN IODIDE
- Synonyms
- ICN;CNI;cN1A;cN-IA;NT5C1A;Jodcyan;IODOCYANIDE;Cyano iodide;IODINE CYANIDE;CYANOGEN IODIDE
- CBNumber:
- CB3345368
- Molecular Formula:
- CIN
- Molecular Weight:
- 152.92
- MOL File:
- 506-78-5.mol
- Modify Date:
- 2024/3/14 15:18:26
| Melting point | 146 °C |
|---|---|
| Boiling point | 154.34°C (rough estimate) |
| Density | 1.840 |
| storage temp. | Refrigerator (+4°C) |
| solubility | soluble in H2O, ethanol, ethyl ether |
| form | Crystalline Powder |
| color | Beige to light brown or light pink |
| Water Solubility | soluble H2O, EtOH, eth [CRC10] |
| Exposure limits | Because cyanogen iodide is a solid with very low vapor pressure, it does not present any inhalation hazard. |
| EPA Substance Registry System | Cyanogen iodide (506-78-5) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS06 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H331-H315-H301-H319-H335-H311 | |||||||||
| Precautionary statements | P261-P271-P304+P340-P311-P321-P403+P233-P405-P501-P264-P280-P305+P351+P338-P337+P313P-P264-P280-P302+P352-P321-P332+P313-P362-P264-P270-P301+P310-P321-P330-P405-P501-P280-P302+P352-P312-P322-P361-P363-P405-P501 | |||||||||
| RIDADR | 3290 | |||||||||
| HazardClass | 6.1(a) | |||||||||
| PackingGroup | I | |||||||||
| HS Code | 28530090 | |||||||||
| NFPA 704 |
|
CYANOGEN IODIDE price More Price(5)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | SAB4503371 | Anti-NT5C1A antibody produced in rabbit affinity isolated antibody | 506-78-5 | 100μG | ₹50560.5 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | SAB2107514 | Anti-NT5C1A antibody produced in rabbit affinity isolated antibody | 506-78-5 | 100μL | ₹47940.9 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | SAB1303669 | ANTI-5NT1A (N-TERM) antibody produced in rabbit IgG fraction of antiserum, buffered aqueous solution | 506-78-5 | 400μL | ₹47796.6 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | HPA054158 | Anti-NT5C1A antibody produced in rabbit Prestige Antibodies? Powered by Atlas Antibodies, affinity isolated antibody, buffered aqueous glycerol solution | 506-78-5 | 100μL | ₹41436.3 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | HPA050283 | Anti-NT5C1A antibody produced in rabbit Prestige Antibodies? Powered by Atlas Antibodies, affinity isolated antibody, buffered aqueous glycerol solution | 506-78-5 | 100μL | ₹41436.3 | 2022-06-14 | Buy |
CYANOGEN IODIDE Chemical Properties,Uses,Production
Chemical Properties
Iodine cyanide (cyanogen iodide) appears as light pink crystalline or brown-coloured powder and is soluble in water. It is stable but sensitive to light. It is incompatible with strong acids, strong bases, and strong oxidising agents. Cyanogen iodide decomposes on contact with acids, bases, and ammonia alcohols and on heating producing toxic gases including hydrogen cyanide. It reacts with carbon dioxide or slowly with water to produce hydrogen cyanide.
Uses
Generally for destroying all lower forms of life. In taxidermy for preserving insects, butterflies, etc.
General Description
White needles with a very pungent odor. Used in taxidermists' preservatives and generally for destroying all lower forms of life. Toxic by inhalation or ingestion.
Air & Water Reactions
Water soluble.
Reactivity Profile
Phosphorus(molten) plus CYANOGEN IODIDE reacts with incandescence to produce phosphorus iodide, [NFPA 491M, 1991]. Benzene and cyanogen halides yield HCl as a byproduct (Hagedorn, F. H. Gelbke, and Federal Republic of Germany. 2002. Nitriles. In Ullman Encyclopedia of Industrial Chemistry. Wiley-VCH Verlag GmbH & Co. KGaA.).
Health Hazard
Cyanogen bromide is a highly poisonous substance. Toxic routes are oral intake and skin absorption. Acute toxic symptoms on test animals were convulsion, paralysis, and respiratory failure. Ingestion of a 5-g amount could be fatal to humans.
LDLo value, oral (cats): 18 mg/kg
LDLo value, subcutaneous (dogs): 19 mg/kg
Cyanogen iodide is an irritant to skin.
Fire Hazard
When heated to decomposition, CYANOGEN IODIDE emits very toxic fumes of nitrogen oxides, cyanide, and iodide. Avoid phosphorus.
Safety Profile
A poison by ingestion and subcutaneous routes. Violent reaction with P. See other cyanogen entries; CYANIDE and IOdiDES. When heated to decomposition it emits very toxic fumes of NOx, CN-, and I-
Potential Exposure
Reacts slowly with water releasing hydrogen cyanide. Incompatible with phosphorus (molten); reacts with incandescence to produce phosphorus iodide . Contact with alcohols, acids, ammonia, carbon dioxide or alkaline material and bases produces toxic gases including hydrogen cyanide. Incompatible with nitriles.
Shipping
UN2928 Toxic solids, corrosive, organic, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials, 8-Corrosive material, Technical Name Required. UN3290 Toxic solid, corrosive, inorganic, n.o.s., Hazard class: 6.1; Labels: 6.1-Poisonous materials, 8-Corrosive material. UN1588 Cyanides, inorganic, solid, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials
Purification Methods
This compound is POISONOUS, and the precautions for cyanogen bromide (above) apply here. The reagent (ca 5.9g) is dissolved in boiling CHCl3 (15mL), filtered through a plug of glass wool into a 25mL Erlenmeyer flask. Cool to room temperature for 15minutes, then place it in an ice-salt bath and cool to -10o. This cooling causes a small aqueous layer to separate as ice. The ice is filtered with the CNI, but melts on the filter and is also removed with the CHCl3 used as washing liquid. The CNI which is collected on a sintered glass funnel is washed 3x with CHCl3 (1.5mL at 0o) and freed from last traces of solvent by placing it on a watch glass and exposing it to the atmosphere in a good fume cupboard at room temperature for 1hour to give colourless needles (ca 4.5g), m 146-147o (sealed capillary totally immersed in the oil bath). The yield depends slightly on the rapidity of the operation; in this way loss by sublimation can be minimised. If desired, it can be sublimed under reduced pressure at temperatures at which CNI is only slowly decomposed into I2 and (CN)2. The vacuum will need to be renewed constantly due to the volatility of CNI. [Bak & Hillebert Org Synth Coll Vol IV 207 1963.]
Incompatibilities
Reacts slowly with water releasing hydrogen cyanide. Incompatible with phosphorus (molten); reacts with incandescence to produce phosphorus iodide . Contact with alcohols, acids, ammonia, carbon dioxide or alkaline material and bases produces toxic gases including hydrogen cyanide. Incompatible with nitriles.
Waste Disposal
A suitable method for destroying cyanogen iodide may consist of treatment with caustic soda, followed by adding sodium hypochlorite (laundry bleach) to oxidize the cyanide to nontoxic cyanate.
CYANOGEN IODIDE Preparation Products And Raw materials
Raw materials
1of2
chevron_rightPreparation Products
506-78-5(CYANOGEN IODIDE)Related Search:
1of2
chevron_right




