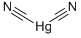
MERCURIC CYANIDE
- CAS No.
- 592-04-1
- Chemical Name:
- MERCURIC CYANIDE
- Synonyms
- CIANURINA;dicyanomercury;MERCURY CYANIDE;mercurydicyanide;cyanuredemercure;MERCURIC CYANIDE;Dicyanomercury(II);Cyanure de mercure;MERCURY(+2)CYANIDE;MERCURY(II) CYANIDE
- CBNumber:
- CB5204793
- Molecular Formula:
- C2HgN2
- Molecular Weight:
- 252.62
- MOL File:
- 592-04-1.mol
- Modify Date:
- 2024/7/10 13:29:25
| Melting point | 46.85°C |
|---|---|
| Density | 3.996 g/mL at 25 °C(lit.) |
| storage temp. | Poison room |
| solubility | Methanol (Slightly), THF (Soluble) |
| form | Fine Crystalline Powder |
| color | White |
| Specific Gravity | 3.996 |
| Water Solubility | g/100g solution H2O: 6.31 (0°C), 10.06±0.06 (25°C), 35.05 (101.1°C) [KRU93]; 1g dissolves in 13mL alcohol, 4mL methanol; slightly soluble ether; slowly soluble glycerol [MER06] |
| Merck | 13,5903 |
| BRN | 4652800 |
| Exposure limits | TLV-TWA 0.1 mg Hg/m3 (skin) (ACGIH). |
| Stability | Acid Sensitive, Light Sensitive |
| CAS DataBase Reference | 592-04-1(CAS DataBase Reference) |
| EPA Substance Registry System | Mercuric cyanide (592-04-1) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS06, GHS08, GHS09 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H300+H310+H330-H373-H410 | |||||||||
| Precautionary statements | P260-P280-P301+P310+P330-P302+P352+P310-P304+P340+P310-P403+P233 | |||||||||
| Hazard Codes | T+,N | |||||||||
| Risk Statements | 26/27/28-32-50/53 | |||||||||
| Safety Statements | 7-28-29-45-60-61 | |||||||||
| RIDADR | UN 1636 6.1/PG 2 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | OW1515000 | |||||||||
| F | 8 | |||||||||
| HazardClass | 6.1(a) | |||||||||
| PackingGroup | II | |||||||||
| HS Code | 28521000 | |||||||||
| NFPA 704 |
|
MERCURIC CYANIDE Chemical Properties,Uses,Production
Chemical Properties
Mercuric cyanide is an odorless, white crystalline solid; turns gray to dark brown when exposed to light
Uses
Mercuric cyanide finds veterinary application as a topical antiseptic for cats and other animals.
Preparation
One part of HgO is digested for a few hours on a water bath with one part of Prussian blue and 10 parts of H2O. The mercuric cyanide crystals separate on evaporation of the solution.
General Description
Odorless tetragonal crystals or white powder. Toxic by inhalation (dust, and the hydrogen cyanide from decomposition) and by ingestion. Toxic oxides of nitrogen are produced in fires. It is used in medicine, germicidal soaps, photography, and in making cyanogen gas.
Air & Water Reactions
Soluble in water. Gradually decomposed by water to give off hydrogen cyanide, a flammable poison gas.
Reactivity Profile
MERCURIC CYANIDE is rapidly decomposed by acids to give off hydrogen cyanide, a flammable poison gas. Decomposed in the light. May tend to explosive instability. Capable of violent reaction with oxidizing agents. Fusion with metal chlorates, perchlorates, nitrates or nitrites can cause a violent explosion [Bretherick 1979. p. 101].
Hazard
Toxic by ingestion, inhalation, and skin absorption.
Health Hazard
Mercuric cyanide is a highly poisonous compound. Its components, mercury(II) and the cyanide ions, are both highly toxic. Its toxicity, however, is lower than that of sodium and potassium cyanides.
Acute toxic symptoms from oral intake of this compound in humans are hypermotility, diarrhea, nausea or vomiting, and injury to kidney and bladder. Toxic symptoms may be manifested in humans from consuming 15–20 g of this compound. Lower doses may produce somnolence. An intraperitoneal dosage of 7.5 mg/kg was fatal to rats.
LD50 value, oral (mice): 7.5 mg/kg.
Fire Hazard
Special Hazards of Combustion Products: Fumes from fire may contain toxic mercury and hydrogen cyanide.
Safety Profile
Poison by ingestion, subcutaneous, intravenous, and intraperitoneal routes. Human systemic effects by ingestion: nausea or vomiting, hypermotility, dlarrhea, kidney changes, somnolence. Hydrolyzes to toxic fumes. A frictionand impact-sensitive explosive. It may initiate detonation of liquid hydrogen cyanide. Incompatible with fluorine, magnesium, sodium nitrite. When heated to decomposition it emits very toxic fumes of Hg, NOx, and CN-. See also CYANIDE and MERCURY COMPOUNDS.
Potential Exposure
Mercuric cyanide is used in medicine, germicidal soaps, photography and in making cyanogen gas
Shipping
UN1636 Mercuric cyanide, Hazard Class: 6.1; Labels: 6.1-Poisonous materials
Purification Methods
Crystallise it from water. The solubility in H2O is 8% at ~20o and 33% at ~100o; in EtOH it is 8% at ~20o and in MeOH it is 25% at ~20o. [Blitz Z Anorg Allgem Chem 170 161 1928.] POISONOUS.
Incompatibilities
Violent reaction with fluorine, magnesium, sodium nitrite, acids. Heating or contact with acid releases toxic mercury and flammable hydrogen cyanide gas. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides
Waste Disposal
Return to supplier for mercury recovery and deactivation.





