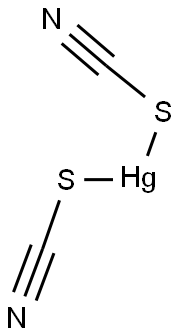
MERCURIC THIOCYANATE
- CAS No.
- 592-85-8
- Chemical Name:
- MERCURIC THIOCYANATE
- Synonyms
- MERCURY(II) THIOCYANATE;MERCURY RHODANIDE;mercurythiocyanate;Mercury(II) thiocyan;MERCURY SULFOCYANIDE;MERCURIC THIOCYANATE;mercuricsulfocyanide;mercurydithiocyanate;Mercury sulfocyanate;MERCURIC SULFOCYANATE
- CBNumber:
- CB0111438
- Molecular Formula:
- C2HgN2S2
- Molecular Weight:
- 316.75
- MOL File:
- 592-85-8.mol
- Modify Date:
- 2024/7/9 8:46:51
| Melting point | 165 °C (dec.) (lit.) |
|---|---|
| Density | 3.71 g/mL at 25 °C (lit.) |
| Flash point | 120 °C |
| storage temp. | Store at RT. |
| solubility | Methanol (Slightly) |
| form | Powder |
| color | White to pale yellow |
| Water Solubility | Soluble in dilute hydrochloric acid, potassium cyanide and ammonia. Slightly soluble in alcohol and ether. Insoluble in water. |
| Sensitive | Hygroscopic |
| Merck | 14,5890 |
| BRN | 3561344 |
| Stability | Stable. Incompatible with strong acids. May be light or moisture sensitive. |
| CAS DataBase Reference | 592-85-8(CAS DataBase Reference) |
| EPA Substance Registry System | Mercuric thiocyanate (592-85-8) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS06,GHS08,GHS09 |
|---|---|
| Signal word | Danger |
| Hazard statements | H300+H310+H330-H373-H410 |
| Precautionary statements | P262-P273-P280-P302+P352+P310-P304+P340+P310-P314 |
| Hazard Codes | T+,N,Xn |
| Risk Statements | 26/27/28-32-33-50/53-20/21/22 |
| Safety Statements | 13-28-45-60-61-46-36/37 |
| RIDADR | UN 1646 6.1/PG 2 |
| WGK Germany | 3 |
| RTECS | XL1550000 |
| F | 8 |
| TSCA | Yes |
| HazardClass | 6.1 |
| PackingGroup | II |
| HS Code | 28521000 |
MERCURIC THIOCYANATE price More Price(14)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | M4128 | Mercury(II) thiocyanate 96.5-103.5% (titration) | 592-85-8 | 25G | ₹6992.95 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | M4128 | Mercury(II) thiocyanate 96.5-103.5% (titration) | 592-85-8 | 100G | ₹21000.5 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 83374 | Mercury(II) thiocyanate purum p.a., ≥95.5% (complexometric) | 592-85-8 | 5G | ₹3052.65 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 83374 | Mercury(II) thiocyanate purum p.a., ≥95.5% (complexometric) | 592-85-8 | 100G | ₹23133.03 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 83374 | Mercury(II) thiocyanate purum p.a., ≥95.5% (complexometric) | 592-85-8 | 500G | ₹81284.93 | 2022-06-14 | Buy |
MERCURIC THIOCYANATE Chemical Properties,Uses,Production
Chemical Properties
Mercuric thiocyanate is an inorganic chemical substance. It is a stable solid at room temperature, and depending upon the purity, it appears as odourless white crystalline powder or grey. It is insoluble in water and denser than water and sinks in water. The solubility in alcohol and boiling H2O and in KSCN solution is higher, in ether lower. Decomposes with swelling on heating to 165°C. On decomposition, mercuric thiocyanate releases hazardous substances such as cyanide vapours, vapours of mercury, oxides of nitrogen (NO, NO2), and oxides of sulphur (SO2, SO3). Mercury thiocyanate has limited uses in chemical synthesis.
Uses
For Pharaoh's serpents (fireworks); intensifier in photography.
Air & Water Reactions
Insoluble in water.
Reactivity Profile
MERCURIC THIOCYANATE decomposes into its elements at about 165°C. Burns readily in air to generate a coil of cohesive ash resembling a serpent (hence used in a firework: Pharaoh's serpents). Swells up to many times its original volume if heated [USCG, 1999]. Soluble in dilute hydrochloric acid [Merck]. Incompatible with strong oxidizing agents.
Hazard
Highly toxic by ingestion, inhalation, and skin absorption.
Health Hazard
Mercuric thiocyanate causes severe eye and skin irritation with possible burns and causes digestive and respiratory tract irritation with possible burns. It may impair fertility, may cause harm to the unborn child, is harmful if inhaled, may cause allergic skin reaction, may cause kidney damage, may cause CNS effects, is light sensitive, and is a severe marine pollutant. Contact with acids liberates very toxic gas. The target organs include kidneys, CNS, reproductive system, eyes, and skin.
Safety Profile
A poison by ingestion and intraperitoneal routes. Moderately toxic by skin contact. Thermally unstable and decomposition may be vigorous. When heated to decomposition it emits very toxic fumes of Hg, NOx, SOx, and CN-. See also MERCURY COMPOUNDS and CYANATES.
Synthesis
A Hg(NO3)2
solution, acidified with a few drops of HNO3,
is treated with the stoichiometric amount of KSCN solution. The
resultant crystalline precipitate is suction-filtered and washed
with H2O. The product may be recrystallized from hot H2O or
alcohol. Yield 80%.
Hg(NO3)2 + 2 KSCN = Hg(SCN)2 + 2KNO3
Potential Exposure
Mercury thiocyanate is used in photography and fireworks.
Shipping
UN1646 Mercury thiocyanate, Hazard Class: 6.1; Labels: 6.1-Poisonous materials.
Purification Methods
Recrystallise it from H2O, and it can give various crystal forms depending on conditions. Its solubility in H2O is 0.069% at 25o, but is more soluble at higher temperatures. It decomposes to Hg above 165o. Poisonous. [Mason & Forgeng J Phys Chem 35 1121 1931, Birckenbach & Kolb Chem Ber 68 919 1935.]
Incompatibilities
Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides. Mercury thiocyanate is sensitive to heat; expands to many times its original volume and then decomposes at freezing/melting point forming toxic fumes of sulfur oxides, mercury cyanide, and nitrogen oxides. Contact with acid or acid fumes causes release of toxic mercury and cyanide vapors. Incompatible with chlorine, reducing agents such as hydrides, sulfides
Waste Disposal
Small amounts may be destroyed by alkaline hydrolysis. Admixture with alkali can be followed by soil burial. Larger quantities can be disposed of by incineration in admixture with acetone or xylene and using effluent gas scrubbing. Do not reuse empty container; proper disposal required.
MERCURIC THIOCYANATE Preparation Products And Raw materials
Raw materials
Preparation Products
592-85-8(MERCURIC THIOCYANATE)Related Search:
1of2
chevron_right




