DIMETHYLMERCURY
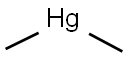
- CAS No.
- 593-74-8
- Chemical Name:
- DIMETHYLMERCURY
- Synonyms
- (CH3)2Hg;imethylmercury;dimethyl-mercur;DIMETHYLMERCURY;methylmercury II;MERCURY DIMETHYL;MONOMETHYLMERCURY;Methyl mercury ion;Dimethylmercury,98%;Dimethylmercury(II)
- CBNumber:
- CB3195062
- Molecular Formula:
- C2H6Hg
- Molecular Weight:
- 230.66
- MOL File:
- 593-74-8.mol
- Modify Date:
- 2024/12/18 14:08:57
| Melting point | −43 °C(lit.) |
|---|---|
| Boiling point | 93-94 °C(lit.) |
| Density | 2.961 g/mL at 25 °C(lit.) |
| refractive index |
n |
| Flash point | 42 °F |
| storage temp. | Flammables area |
| solubility | insoluble in H2O; very soluble in ethanol, ethyl ether |
| form | liquid |
| Water Solubility | insoluble H2O; soluble ether, alcohol [MER06] |
| Merck | 13,3276 |
| BRN | 3600205 |
| Exposure limits |
TLV-TWA: 0.01 mg (Hg)/m3 (ACGIH) PEL-TWA: 0.01 mg (Hg)/m3 (OSHA) STEL: 0.03 mg (Hg)/m3 (ACGIH) The tolerable weekly intake (TWI) levels set by World Health Organization for methyl mercury is 1.6 μg/kg body weight. The reference dose (RfD) set by the U.S. EPA is 0.1 μg/kg body weight/day (Booth and Zeller (2005). |
| Stability | Stable. Highly flammable. Incompatible with strong oxidizing agents. |
| EPA Substance Registry System | Dimethylmercury (593-74-8) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS06,GHS08,GHS09 |
|---|---|
| Signal word | Danger |
| Hazard statements | H300+H310+H330-H373-H410 |
| Precautionary statements | P262-P273-P280-P301+P310+P330-P302+P352+P310-P304+P340+P310 |
| Hazard Codes | T+,N |
| Risk Statements | 26/27/28-33-50/53 |
| Safety Statements | 13-28-36-45-60-61 |
| RIDADR | UN 3383 6.1/PG 1 |
| WGK Germany | 3 |
| RTECS | OW3010000 |
| HazardClass | 3.1 |
| PackingGroup | II |
DIMETHYLMERCURY Chemical Properties,Uses,Production
Description
The first indication of the extreme toxicity of dimethylmercury (DMM) was documented in 1863 when two laboratory assistants died of DMM poisoning while synthesizing DMM in the laboratory of Frankland and Duppa. There are numerous reports of people dying from alkyl mercury compounds including a chemist who was preparing several thousand grams ofDMMin his laboratory in 1974. The extreme toxicity was revisited in 1997, when Karen Wetterhahn, an internationally renowned researcher of the carcinogenic effects of heavy metals on DNA repair proteins, died within a few months after a single exposure of less than a milliliter of DMM on her latex-covered hand. DMM is extremely toxic and lethal at a dose of approximately 400 mg of mercury (equivalent to a few drops) or about 5mgkg-1 of body weight or as little as 0.1 ml
Chemical Properties
colourless liquid
Uses
Dimethylmercury is used as a reagent ininorganic synthesis, and as a reference standardfor mercury nuclear magnetic resonance(Hg NMR). It is an environmental pollutantfound in bottom sediments and also inthe bodies of birds and marine mammalssuch as whales and fishes. It occurs in fishesand birds along with monomethylmercury. Inhumans, its presence is attributed to the consumptionof pilot whale meat, cod fish, andother sea food.
Health Hazard
All alkylmercury compounds are highly toxicby all routes of exposure. There are manyserious cases of human poisoning frommethylmercury (Lu 2003). Outbreaks ofmass poisoning from consumption of contaminatedfish occurred in Japan during the1950s, causing a severe neurological disease,so-called “Minamata disease,” whichresulted in hundreds of deaths. A similaroutbreak of food poisoning from contaminatedwheat caused several hundred deathsin Iraq in 1972. A tragic death from a singleacute transdermal exposure to dimethylmercury(estimated between 0.1 to 0.5 mL) thatpenetrated into the skin through disposablelatex gloves has occurred (Blayney et al.1997; The New York Times, June 11, 1997).The symptoms reported were episodes ofnausea and vomiting occurring three monthsafter the exposure followed by onset ofataxia, slurred speech (dysarthia), and loss ofvision and hearing 2 months after that. Thedeath occurred in about six months after theaccident.
Methylmercury can concentrate in certainfetal organs, such as the brain. Thetarget organs are the brain and the centralnervous system. It can cause death, miscarriage,and deformed fetuses. Unlike inorganicmercury compounds, it can penetrate throughthe membrane barrier of the erythrocyte,accumulating at about 10 times greater concentrationthan that in the plasma (WHO1976). Its rate of excretion on the bloodlevel is very slow. It gradually accumulatesin the blood. Such accumulation was found toreach 60% equilibrium at about 90 days, culminatedafter 270 days (Munro and Willes,1978). Skin absorption exhibits the symptomsof mercury poisoning. The toxic thresholdconcentration of mercury in the wholeblood is usually in the range 40 to 50 μg/L,while the normal range should be below10 μg/L.
Fire Hazard
It is a flammable liquid; flash point 38°C (101°F). The flammability of this compound, its ease of oxidation and the energy of decomposition is relatively lower than the alkyls of lighter metals. It is mildly endothermic. The heat of formation, △H°f is +75.3 kJ/mol (Bretherick 1995). Unlike most other metal alkyls formed by elements of lower atomic numbers, dimethylmercury does not pose any serious fire or explosion hazard. Although it does not ignite in air, the compound is easily inflammable. It dissolves in lower alcohols without any violent decomposition. Heating with oxidizing substances can cause explosion. Violent explosion is reported with diboron tetrachloride at -63°C (-81°F) under vacuum (Wartik et al. 1971).
Safety Profile
Suspected carcinogen. Highly toxic. Mutation data reported. Easily flammable. When heated to decomposition it emits toxic fumes of Hg.
Potential Exposure
Dimethyl mercury has been used as seed disinfectants and for fungicides. It has also been used in organic synthesis.
Environmental Fate
DMM is a colorless liquid that is volatile at room temperature (vapor pressure 62.3 mmHg) and is slightly soluble in water (water solubility 8860 mg l-1). There are no reports on the partition behavior of DMM but it is known to readily evaporate and is thus rarely found in sediment or soil. No reports were found on the environmental persistence of DMM. While DMM vaporizes, no studies were found on long range transport. The lipophilicity ofDMMresults in its accumulation inadipose tissue, plasma proteins, and brain. DMM has not been found in fish.
Shipping
UN2025 Mercury compounds, solid, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials, Technical Name Required.
Incompatibilities
Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides. May be sensitive to light.
DIMETHYLMERCURY Preparation Products And Raw materials
Raw materials
1of2
chevron_rightPreparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Singhvi | 08048372519Ext 763 | Mumbai, India | 1 | 58 | Inquiry |
| Shanghai Longyu Biotechnology Co., Ltd. | +8619521488211 | China | 2555 | 58 | Inquiry |
| Shaanxi Dideu Medichem Co. Ltd | +86-29-87569266 15319487004 | China | 3978 | 58 | Inquiry |
| RongNa Biotechnology Co.,Ltd | +86-86-13583358881 +8618560316533 | China | 3084 | 58 | Inquiry |
| GIHI CHEMICALS CO.,LIMITED | +8618058761490 | China | 49984 | 58 | Inquiry |
| Henan Bao Enluo International TradeCo.,LTD | +86-17331933971 +86-17331933971 | China | 2472 | 58 | Inquiry |
| ABCR GmbH & CO. KG | 49 721 95061 0 | Germany | 6831 | 75 | Inquiry |
| Kanto Chemical Co., Inc. | +81 3 3663 7631 | Japan | 6756 | 74 | Inquiry |
| Pfaltz & Bauer, Inc. | (800) 225-5172 | United States | 6828 | 72 | Inquiry |
| MP Biomedicals, Inc. | 949 833 2500 | United States | 6561 | 81 | Inquiry |
Related articles
- Use and Environmental Fate of dimethylmercury
- The first indication of the extreme toxicity of dimethylmercury (DMM) was documented in 1863 when two laboratory assistants di....
- Jan 19,2022
- Toxcity of Dimethylmercury
- DMM has limited use because of its toxicity but can be used to calibrate research equipment, as in its application as a standa....
- Oct 13,2021





