Sulfacarbamide
- CAS No.
- 547-44-4
- Chemical Name:
- Sulfacarbamide
- Synonyms
- a435;A 435;Uramid;Urenil;Uractyl;Euvernil;Sulfaurea;Urosulfan;NSC 78438;Sulphaurea
- CBNumber:
- CB3711796
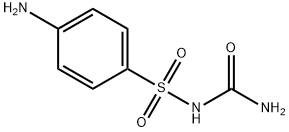
- Molecular Formula:
- C7H9N3O3S
- Molecular Weight:
- 215.23
- MOL File:
- 547-44-4.mol
- MSDS File:
- SDS
- Modify Date:
- 2024/11/20 11:41:24
| Melting point | 146-148° (slight dec) |
|---|---|
| Density | 1.4565 (rough estimate) |
| refractive index | 1.6630 (estimate) |
| storage temp. | under inert gas (nitrogen or Argon) at 2–8 °C |
| solubility | DMSO : ≥ 28 mg/mL (130.09 mM) |
| form | Solid |
| pka | pKa 1.78(H2O t = 25 I = 0.05) (Uncertain);5.42(H2O t = 25 I = 0.05) (Uncertain) |
| color | White to off-white |
| Water Solubility | 2.333g/L(20 ºC) |
| InChI | InChI=1S/C7H9N3O3S/c8-5-1-3-6(4-2-5)14(12,13)10-7(9)11/h1-4H,8H2,(H3,9,10,11) |
| InChIKey | WVAKABMNNSMCDK-UHFFFAOYSA-N |
| SMILES | C1(S(NC(N)=O)(=O)=O)=CC=C(N)C=C1 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|---|---|
| Signal word | Warning |
| Hazard statements | H317-H302 |
| Precautionary statements | P261-P272-P280-P302+P352-P333+P313-P321-P363-P501-P264-P270-P301+P312-P330-P501 |
Sulfacarbamide Chemical Properties,Uses,Production
Description
Sulfacarbamide is a blood sugar-lowering drug, also acting on the vegetative nervous system.
Chemical Properties
White solid
Uses
antibacterial
World Health Organization (WHO)
Sulfacarbamide, a sulfonamide anti-infective agent, was introduced in the 1940's for the treatment of bacterial infections. The importance of sulfonamides has subsequently decreased as a result of increasing bacterial resistance and their replacement by antibiotics which are generally more active and less toxic. The Sulfacarbamide are known to cause serious adverse effects such as renal toxicity, sometimes fatal exfoliative dermatitis and erythema multiforma and dangerous adverse reactions affecting blood formation such as agranulocytosis and haemolytic or aplastic anaemia. Sulfacarbamide still remains available in at least one country for the treatment of urinary infections.
Mechanism of action
Sulfonylureas bind to and inhibit the ATP-sensitive potassium channels (K) on the pancreatic beta cells. As a result, potassium efflux decreases, and the beta-cell membrane depolarizes. Membrane depolarization causes calcium channels to open, leading to calcium influx and increased intracellular calcium, which stimulates insulin secretion from the pancreatic beta cells. Sulfonylureas cause insulin release regardless of blood glucose levels.
Sulfacarbamide Preparation Products And Raw materials
Sulfacarbamide Suppliers
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Pharmaffiliates Analytics and Synthetics P. Ltd | +91-172-5066494 | Haryana, India | 6739 | 58 | Inquiry |
| Finetech Industry Limited | +86-27-8746-5837 +8619945049750 | China | 9642 | 58 | Inquiry |
| TargetMol Chemicals Inc. | +1-781-999-5354 +1-00000000000 | United States | 32159 | 58 | Inquiry |
| Dayang Chem (Hangzhou) Co.,Ltd. | +86-0571-88938639 +8617705817739 | China | 52849 | 58 | Inquiry |
| Shaanxi Didu New Materials Co. Ltd | +86-89586680 +86-13289823923 | China | 8772 | 58 | Inquiry |
| TargetMol Chemicals Inc. | United States | 38630 | 58 | Inquiry | |
| GIHI CHEMICALS CO.,LIMITED | +8618058761490 | China | 49984 | 58 | Inquiry |
| LEAPCHEM CO., LTD. | +86-852-30606658 | China | 43340 | 58 | Inquiry |
| Nanjing Bicbiotechnology Co., Ltd | +86-2552131256 +86-18251840740 | China | 5994 | 58 | Inquiry |
| TargetMol Chemicals Inc. | +8613564774135 | United States | 19881 | 58 | Inquiry |
547-44-4(Sulfacarbamide)Related Search:
1of4
chevron_right




