DIALLATE
- CAS No.
- 2303-16-4
- Chemical Name:
- DIALLATE
- Synonyms
- DATC;Avadex;cp15336;Diallat;Pyradex;2,3-DCDT;Avadex I;DIALLATE;CP 15336;cp15,336
- CBNumber:
- CB3751152
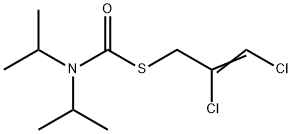
- Molecular Formula:
- C10H17Cl2NOS
- Molecular Weight:
- 270.22
- MOL File:
- 2303-16-4.mol
- MSDS File:
- SDS
- Modify Date:
- 2024/7/2 8:55:06
| Melting point | 25-30℃ |
|---|---|
| Boiling point | bp9 150° |
| Density | 1.1556 (rough estimate) |
| refractive index | 1.6000 (estimate) |
| Flash point | >100 °C |
| storage temp. | 0-6°C |
| form | Viscous Liquid |
| pka | -1.63±0.70(Predicted) |
| color | Colorless to light yellow |
| Water Solubility | 40mg/L(room temperature) |
| IARC | 3 (Vol. 30, Sup 7) 1987 |
| EPA Substance Registry System | Diallate (2303-16-4) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS08,GHS07,GHS09 |
|---|---|
| Signal word | Warning |
| Hazard statements | H302-H351-H410 |
| Precautionary statements | P264-P270-P301+P312-P330-P501-P201-P202-P281-P308+P313-P405-P501-P273-P391-P501 |
| Hazard Codes | Xn,N |
| Risk Statements | 22-40-50/53 |
| Safety Statements | 25-36/37-60-61 |
| RIDADR | 2902 |
| RTECS | EZ8225000 |
| HazardClass | 6.1(b) |
| PackingGroup | III |
| HS Code | 29302000 |
| Toxicity | LD50 orally in rats: 395 mg/kg (Bailey, White) |
DIALLATE Chemical Properties,Uses,Production
Chemical Properties
Diallate is a brown liquid.
Uses
Diallate is a pesticide used to treat fruits and vegetables. Environmental wastewater pollutant.Environmental toxin on US EPA Toxic Release Inventory list (TRI) list.
General Description
Used as an herbicide.
Air & Water Reactions
Thio and dithiocarbamates slowly decompose in aqueous solution to form carbon disulfide and methylamine or other amines. Such decompositions are accelerated by acids.
Reactivity Profile
DIALLATE is a thiocarbamate ester. Flammable gases are generated by the combination of thiocarbamates and dithiocarbamates with aldehydes, nitrides, and hydrides. Thiocarbamates and dithiocarbamates are incompatible with acids, peroxides, and acid halides.
Safety Profile
Poison by ingestion. Moderately toxic by skin contact. Questionable carcinogen with experimental carcinogenic and tumorigenic data. Mutation data reported. When heated to decomposition it emits very toxic fumes of Cl-, NOx, and SOx. See also CARBAMATES and ALLYL COMPOUNDS.
Potential Exposure
A thiocarbamate herbicide. The slow release of poisonous gases from hydrolysis of many thio and dithiocarbamates requires the use of respirators during handling. Poisoning can also occur by ingestion and absorption through the skin. Diallate is potential danger to those involved in the manufacture, formulation and applica- tion of this re-emergence herbicide.
Environmental Fate
Soil. Soil metabolites include 2,3-dichloroallyl alcohol, 2,3-dichloroallyl mercaptan
(Hartley and Kidd, 1987) and carbon dioxide (Smith, 1988). The formation of the alcohol
occurs via hydrolysis of the ester linkage and transthiolation of the allylic group (Kaufman,
1967). In an agricultural soil, 14CO2 was the only biodegradation identified; however, bound
residue and traces of benzene and water-soluble radioactivity were also detected in large
amounts (Anderson and Domsch, 1980). The reported half-lives in soil range from 2 to 4
weeks to (Smith and Fitzpatrick, 1970) to approximately 30 days (Hartley and Kidd, 1987).
Diallate did not migrate deeper than 5 cm on test field plots (Smith, 1970). In four
microbially-active agricultural soils, the half-life was 4 weeks but in sterilized soil the
half-life was 20 weeks (Anderson and Domsch, 1976).
Plant. In plants, diallate is metabolized and carbon dioxide is released (Hartley and
Kidd, 1987). Diallate undergoes cis/trans isomerization and oxidative cleavage when
irradiated at 300 nm forming 2,3-dichloroacrolein and 2-chloroacrolein as products (Ruzo
and Casida, 1985).
Photolytic. Irradiation of diallate was also conducted in oxygenated chloroform or
water until a 10% conversion was obtained. Products formed included acetaldehyde, 2,3-
dichloroacrolein and trace amounts of 2-chloroacrolein (Ruzo and Casida, 1985).
Chemical/Physical. Emits toxic fumes of chlorine, nitrogen and sulfur oxides when
heated to decomposition (Sax and Lewis, 1987). Though no products were reported, the
calculated hydrolysis half-life at 25°C and pH 7 is 6.6 years (Ellington et al., 1988).
Shipping
UN2992 Carbamate pesticides, liquid, toxic, Hazard Class: 6.1; Labels: 6.1-Poisonous materials.
Incompatibilities
hiocarbamate esters are combustible. They react violently with powerful oxidizers such as calcium hypochlorite. Poisonous gases are generated by the thermal decomposition of thiocarbamate compounds, including carbon disulfide, oxides of sulfur, oxides of nitro- gen, hydrogen sulfide, ammonia, and methylamine. Many materials in this group slowly decompose in aqueous solu- tion to form carbon disulfide and methylamine or other amines. Such decompositions are accelerated by acids. Flammable gases are generated by the combination of thiocarbamates with aldehydes, nitrides, and hydrides. Thiocarbamates are incompatible with acids, peroxides, and acid halides.
Waste Disposal
Land burial is acceptable for small quantities. Larger quantities can be incinerated . In accordance with 40CFR165, follow recommendations for the disposal of pesticides and pesticide containers. Must be disposed properly by following package label directions or by contacting your local or federal environmental control agency, or by contacting your regional EPA office. Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal.
DIALLATE Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| CLEARSYNTH LABS LTD. | +91-22-45045900 | Hyderabad, India | 6351 | 58 | Inquiry |
| Chongqing Chemdad Co., Ltd | +86-023-6139-8061 +86-86-13650506873 | China | 39916 | 58 | Inquiry |
| Career Henan Chemica Co | +86-0371-86658258 +8613203830695 | China | 30253 | 58 | Inquiry |
| Zhuoer Chemical Co., Ltd | 02120970332; +8613524231522 | China | 3005 | 58 | Inquiry |
| Hebei Duling International Trade Co. LTD | +8617333973358 | China | 15437 | 58 | Inquiry |
| GIHI CHEMICALS CO.,LIMITED | +8618058761490 | China | 49999 | 58 | Inquiry |
| LGC Standards | +44 (0)20 8943 8480 | United Kingdom | 6495 | 0 | Inquiry |
| Shaanxi Didu New Materials Co. Ltd | +86-89586680 +86-13289823923 | China | 9003 | 58 | Inquiry |
| Hubei Guangao Biotechnology Co., Ltd | 027-027-59223056 18162699093 | China | 9928 | 58 | Inquiry |
| Hubei yongkuo Technology Co., Ltd | 027-59223108 15972152991 | China | 9993 | 58 | Inquiry |
2303-16-4(DIALLATE)Related Search:
1of2
chevron_right




