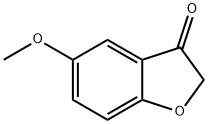
5-Methoxybenzofuran-3(2H)-one
- CAS No.
- 39581-55-0
- Chemical Name:
- 5-Methoxybenzofuran-3(2H)-one
- Synonyms
- AKOS BC-0911;5-METHOXY-BENZOFURAN-3-ONE;5-Nitro-3-Benzofuranone (2);5-methoxy-1-benzofuran-3-one;5-methoxybenzofuran-3(2H)-one;5-Methoxy-3(2H)-benzofuranone;3(2H)-Benzofuranone, 5-Methoxy-;5-Methoxy-1-benzofuran-3(2H)-one;1H-1,4-Diazepine,hexahydro-4-(phenylmethyl)-
- CBNumber:
- CB3840863
- Molecular Formula:
- C9H8O3
- Molecular Weight:
- 164.16
- MOL File:
- 39581-55-0.mol
- Modify Date:
- 2024/6/13 16:09:12
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|---|---|
| Signal word | Warning |
| Hazard statements | H302-H315-H319-H335 |
| Precautionary statements | P261-P305+P351+P338 |
5-Methoxybenzofuran-3(2H)-one Chemical Properties,Uses,Production
Synthesis
5-Methoxybenzofuran-3(2H)-one is synthesised using 2-chloro-1-(2-hydroxy-5-methoxyphenyl)ethanone as raw material by chemical reaction. The specific synthesis steps are as follows:
To an ice-cooled solution of boron trichloride (1.2 equiv., 10 mmol, 10 ml of a solution of 1M BC13 in dichloromethane) under N2-atmosphere was added dropwise a solution of J (1 g, 8 mmol,) in dichloromethane (5 ml). Then, chloroacetonitrile (0.7 g, 10 mmol, 1.2 equiv. ) was added dropwise, followed by aluminum(III) chloride (0.5 g, 4 mmol, 0.5 equiv. ) in one portion. The reaction mixture was allowed to warm up to room temperature and was stirred for 6 h. The reaction mixture was diluted with dichloromethane and quenched with IN hydrochloric acid at 0°C. After stirring for 10 min, the aqueous layer was extracted with dichloromethane. The combined organic layers were washed with brine, dried (MgS04) and concentrated Purification by flash chromatography on silica gel (eluent: dichloromethane) afforded the desired product K (1 g, yield = 60percent). Intermediate K (0.15 g, 0.75 mmol, I equiv. ) and potassium acetate (0.22 g, 2.2 mmol, 3 equiv. ) were refluxed in ethanol (10 ml) for 1 h. After cooling, the reaction mixture was filtered and concentrated. The residue was mixed with water, and acidified with IN hydrochloric acid to pH = 1. The aqueous solution was extracted with ethyl acetate, the combined extracts were dried (MgS04), filtered and concentrated to afford the desired product L (110 mg, yield = 90 percent) as a pink solid The experimental procedures for the condensation reaction of compound L with 4-nitroaniline in acetic acid to form compound M, followed by Vilsmeier-Haack formulation and subsequent Knoevenagel condensation of the benzofuran carbaldehyde N with ethyl cyanoacetate to form compound 0, and finally, intramolecular cyclisation to compound 37 were performed using analogous procedures as exemplified in example 1 for the synthesis of compound f starting from compound a. Compound 37 was obtained as a yellow powder (30 mg, purity (LC) = 80 percent).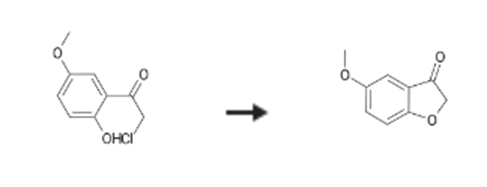
5-Methoxybenzofuran-3(2H)-one Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| A.J Chemicals | 91-9810153283 | New Delhi, India | 6124 | 58 | Inquiry |
| Capot Chemical Co.,Ltd. | 571-85586718 +8613336195806 | China | 29798 | 60 | Inquiry |
| Shenzhen Nexconn Pharmatechs Ltd | +86-755-89396905 +86-15013857715 | China | 10311 | 58 | Inquiry |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | China | 49392 | 58 | Inquiry |
| Shanghai Rlavie Technology Co ltd | +86-021-67759270 +8617717059219 | China | 1030 | 58 | Inquiry |
| Career Henan Chemica Co | +86-0371-86658258 +8613203830695 | China | 30250 | 58 | Inquiry |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418671 +8618949823763 | China | 34571 | 58 | Inquiry |
| Amadis Chemical Company Limited | 571-89925085 | China | 131980 | 58 | Inquiry |
| Taizhou Nanfeng Pharmaceutical Research Institute | 18616377689 | China | 20566 | 58 | Inquiry |
| BEIJING ECHIRAL MED TECH DVLP | 18010053931 | China | 8298 | 58 | Inquiry |





