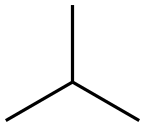
ISOBUTANE
- CAS No.
- 75-28-5
- Chemical Name:
- ISOBUTANE
- Synonyms
- r600a;2-METHYLPROPANE;i-Butane;Isobutan;Methylpropane;methyl-2;tert-Butane;ISOBUTANE HC-600A;Isobutane(incylinderwithoutvalve);R 600a
- CBNumber:
- CB4310831
- Molecular Formula:
- C4H10
- Molecular Weight:
- 58.12
- MOL File:
- 75-28-5.mol
- MSDS File:
- SDS
- Modify Date:
- 2023/6/26 8:28:20
| Melting point | −160 °C(lit.) |
|---|---|
| Boiling point | −12 °C(lit.) |
| Density | 2.064 g/mL at 25 °C(lit.) |
| vapor density | 2.01 (21 °C, vs air) |
| vapor pressure | 72.2 psi ( 37.7 °C) |
| refractive index | 1.3518 |
| Flash point | -83 °C |
| form | Colorless gas |
| color | Colorless, very flammable gas with a faint odor |
| explosive limit | 8.3% |
| Water Solubility | 48.9 mg/kg at 25 °C (shake flask-GC, McAuliffe, 1963, 1966) |
| BRN | 1730720 |
| Henry's Law Constant | 1.171 atm?m3/mol at 25 °C (Hine and Mookerjee, 1975) |
| Exposure limits | NIOSH REL: TWA 800 ppm (1,900 mg/m3). |
| Stability | Stable. Extremely flammable. May form explosive mixtures with air. |
| InChIKey | NNPPMTNAJDCUHE-UHFFFAOYSA-N |
| LogP | 2.760 |
| CAS DataBase Reference | 75-28-5(CAS DataBase Reference) |
| EPA Substance Registry System | Isobutane (75-28-5) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS02,GHS04 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H220-H280 | |||||||||
| Precautionary statements | P410+P403 | |||||||||
| Hazard Codes | F+ | |||||||||
| Risk Statements | 12 | |||||||||
| Safety Statements | 9-16 | |||||||||
| RIDADR | UN 1969 2.1 | |||||||||
| OEB | A | |||||||||
| OEL | TWA: 800 ppm (1900 mg/m3) | |||||||||
| WGK Germany | - | |||||||||
| RTECS | TZ4300000 | |||||||||
| F | 4.5-31 | |||||||||
| Autoignition Temperature | 860 °F | |||||||||
| DOT Classification | 2.1 (Flammable gas) | |||||||||
| HazardClass | 2.1 | |||||||||
| HS Code | 2901100000 | |||||||||
| NFPA 704 |
|
ISOBUTANE Chemical Properties,Uses,Production
Chemical Properties
2-Methylpropane (isobutane), C4H10, a flammable gas, occurs in small quantities in natural gas and crude oil. It has been detected in urban atmospheres at concentrations of 44–74 ppb. It also evolves from natural sources and has been measured in diesel exhaust at 1.4–11 ppm and in cigarette smoke at 10 ppm. The partition coefficient of propane between olive oil and air at 37℃ is 12 using the method described by Sato and Nakajima and Perbellini et al.. The lower explosive limit is 18,000 ppm in air.
Uses
Isobutane occurs in petroleum, natural gas,and petroleum cracking products. It is usedas a fuel gas or a liquefied petroleum gas. Itis also used in organic synthesis.
Definition
ChEBI: An alkane that is propane substituted by a methyl group at position 2.
General Description
ISOBUTANE is a colorless gas with a faint petroleum-like odor. ISOBUTANE is shipped as a liquefied gas under its vapor pressure. Contact with the liquid can cause frostbite. ISOBUTANE is easily ignited. The vapors are heavier than air. Any leak can either be liquid or vapor. ISOBUTANE can asphyxiate by the displacement of air. Under prolonged exposure to fire or intense heat the containers may rupture violently and rocket.
Air & Water Reactions
Highly flammable.
Reactivity Profile
ISOBUTANE is incompatible with the following: Strong oxidizers (e.g., nitrates & perchlorates), chlorine, fluorine, (nickel carbonyl + oxygen) .
Hazard
Highly flammable, dangerous fire and explosive risk; explosive limits in air 1.9–8.5%.
Health Hazard
Isobutane, like other saturated aliphatic alkanes,is nontoxic. It is an asphyxiate. Exposureto high concentrations of 1% in air maycause narcosis and drowsiness. Other thanthis, there is no report of any adverse healtheffect from exposure to this gas.
Fire Hazard
EXTREMELY FLAMMABLE. Will be easily ignited by heat, sparks or flames. Will form explosive mixtures with air. Vapors from liquefied gas are initially heavier than air and spread along ground. CAUTION: Hydrogen (UN1049), Deuterium (UN1957), Hydrogen, refrigerated liquid (UN1966) and Methane (UN1971) are lighter than air and will rise. Hydrogen and Deuterium fires are difficult to detect since they burn with an invisible flame. Use an alternate method of detection (thermal camera, broom handle, etc.) Vapors may travel to source of ignition and flash back. Cylinders exposed to fire may vent and release flammable gas through pressure relief devices. Containers may explode when heated. Ruptured cylinders may rocket.
Safety Profile
An asphyxiant. A common air contaminant. A very dangerous fire and explosion hazard when exposed to heat, flame, or oxidizers. To fight fire, stop flow of gas. When heated to decomposition it emits acrid smoke and irritating fumes.
Environmental Fate
Photolytic. Based upon a photooxidation rate constant of 2.34 x 10-12 cm3/molecule?sec with OH
radicals in summer daylight, the atmospheric lifetime is 59 h (Altshuller, 1991). At atmospheric
pressure and 300 K, Darnall et al. (1978) reported a rate constant of 2.52 x 10-12 cm3/molecule?sec
for the same reaction. Rate constants of 1.28 x 10-9 and 6.03 x 10-12 L/molecule?sec were reported
for the reaction of 2-methylpropane with OH radicals in air at 300 and 296 K, respectively
(Greiner, 1967, 1970). Rate constants of 7.38 x 10-13 and 6.50 x 10-17 cm3/molecule?sec were
reported for the reaction of 2-methylpropane with OH and NO3, respectively (Sablji? and Güsten,
1990).
Chemical/Physical. Complete combustion in air produces carbon dioxide and water vapor. 2-
Methylpropane will not hydrolyze because it does not contain a hydrolyzable functional group.
Purification Methods
Olefins and moisture can be removed by passage at 65o through a bed of silica-alumina catalyst which has previously been evacuated at about 400o. Alternatively, water and CO2 can be removed by passage through P2O5, then asbestos impregnated with NaOH. Treatment with anhydrous AlBr3 at 0o then removes traces of olefins. Inert gases can be separated by freezing the isobutane at -195o and evacuating out the system. [Beilstein 1 IV 282.]
ISOBUTANE Preparation Products And Raw materials
Raw materials
Preparation Products
1of3
chevron_right| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Maharashtra Gas Co | +91-8097713800 +91-9833780675 | Maharashtra, India | 21 | 58 | Inquiry |
| Vadilal Chemicals Limited | +91-7948936937 +91-7203030735 | Gujarat, India | 39 | 58 | Inquiry |
| Bhuruka Gases Ltd | +91-7760976502 +91-8041818200 | Karnataka, India | 11 | 58 | Inquiry |
| CLEARSYNTH LABS LTD. | +91-22-45045900 | Hyderabad, India | 6351 | 58 | Inquiry |
| Imkemex Marketing Private Limited | 08068441148Ext 603 | Mumbai, India | 10 | 58 | Inquiry |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | China | 21661 | 55 | Inquiry |
| Chongqing Chemdad Co., Ltd | +86-023-6139-8061 +86-86-13650506873 | China | 39916 | 58 | Inquiry |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | China | 49403 | 58 | Inquiry |
| career henan chemical co | +86-0371-86658258 +8613203830695 | China | 29820 | 58 | Inquiry |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418671 +8618949823763 | China | 34571 | 58 | Inquiry |
75-28-5(ISOBUTANE)Related Search:
1of4
chevron_right




