NIGERICIN SODIUM SALT
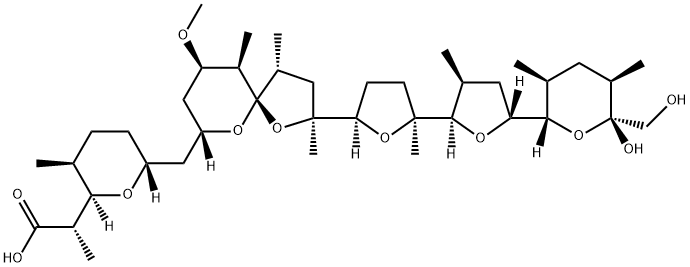
- CAS No.
- 28380-24-7
- Chemical Name:
- NIGERICIN SODIUM SALT
- Synonyms
- x-464;Helix c;HELIXIN C;NIGERICIN;Aids003767;Aids-003767;AZALOMYCIN M;NIGERICIN NA;POLYETHERIN A;Antibiotic M 2
- CBNumber:
- CB4331016
- Molecular Formula:
- C40H68O11
- Molecular Weight:
- 724.96
- MOL File:
- 28380-24-7.mol
- Modify Date:
- 2025/4/17 18:22:24
| Melting point | 183.5-185° |
|---|---|
| alpha | D24 +36.2° (c = 0.842 in CHCl3) |
| Boiling point | 779.9±60.0 °C(Predicted) |
| Density | 1.19±0.1 g/cm3(Predicted) |
| storage temp. | 2-8°C |
| solubility | chloroform: 10 mg/mL, clear, colorless |
| form | Solid |
| pka | 4.39±0.10(Predicted) |
| color | White to off-white |
| InChIKey | DANUORFCFTYTSZ-ANZYULKNSA-N |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS06 |
|---|---|
| Signal word | Danger |
| Hazard statements | H319-H335-H301-H315 |
| Precautionary statements | P264-P270-P301+P310-P321-P330-P405-P501-P264-P280-P302+P352-P321-P332+P313-P362-P264-P280-P305+P351+P338-P337+P313P |
| Hazard Codes | T,Xi |
| Risk Statements | 25-36/37/38 |
| Safety Statements | 26-36/37/39-45-36 |
| RIDADR | UN 3462 6.1/PG 3 |
| WGK Germany | 3 |
| RTECS | QT6840000 |
| F | 10 |
| HazardClass | 6.1(b) |
| PackingGroup | III |
| HS Code | 29419090 |
| Toxicity | LD50 in mice (mg/kg): 10-15 i.p. (Shoji); also reported as 2.5 i.p. (Harned) |
NIGERICIN SODIUM SALT Chemical Properties,Uses,Production
Uses
Nigericin is a polyether antibiotic produced by Streptomyces, notably S. hygroscopicus, isolated in the 1950s. Its complex structure was finally elucidated in 1968. Nigericin is an ionophore, possessing very high affinity for monovalent cations such as Na+ and K+. Nigericin disrupts membrane potential and Golgi apparatus in mitochondria. Although nigericin can be isolated as the free acid (under acidic conditions), like most ionophores it is extracted into organic solvents and is most conveniently isolated as a salt. In vitro, nigericin has broad biological activity against Gram positive bacteria, fungi, tumour cell lines and some viruses, including HIV. Nigericin is the most common member of the polyether class which are common false positives in in vitro screening bioassays using crude microbial extracts. They are thus important standards for dereplication.
Definition
ChEBI: A polyether antibiotic which affects ion transport and ATPase activity in mitochondria. It is produced by Streptomyces hygroscopicus.
NIGERICIN SODIUM SALT Preparation Products And Raw materials
Raw materials
Preparation Products
NIGERICIN SODIUM SALT Suppliers
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| CLEARSYNTH LABS LTD. | +91-22-45045900 | Hyderabad, India | 6257 | 58 | Inquiry |
| Pure Research Chemicals | 13564847951 18019592524 | China | 967 | 58 | Inquiry |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | China | 21628 | 55 | Inquiry |
| career henan chemical co | +86-0371-86658258 +8613203830695 | China | 29808 | 58 | Inquiry |
| SHANGHAI T&W PHARMACEUTICAL CO., LTD. | +86-021-61551413 +8618813727289 | China | 5738 | 58 | Inquiry |
| SIMAGCHEM CORP | +86-13806087780 | China | 17365 | 58 | Inquiry |
| TargetMol Chemicals Inc. | +1-781-999-5354 +1-00000000000 | United States | 32159 | 58 | Inquiry |
| Baoji Guokang Healthchem co.,ltd | +8615604608665 15604608665 | CHINA | 9414 | 58 | Inquiry |
| Hebei Fengjia New Material Co., Ltd | +86-0311-87836622 +86-18712993135 | China | 8051 | 58 | Inquiry |
| LEAP CHEM CO., LTD. | +86-852-30606658 | China | 24727 | 58 | Inquiry |





