ChemicalBook > Product Catalog >Chemical Reagents >Silane reagent >N-ALLYL-N N-BIS(TRIMETHYLSILYL)AMINE
N-ALLYL-N N-BIS(TRIMETHYLSILYL)AMINE
- CAS No.
- 7688-51-9
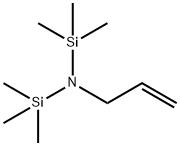
- Chemical Name:
- N-ALLYL-N N-BIS(TRIMETHYLSILYL)AMINE
- Synonyms
- Einecs 231-699-2;Ditrimethylsilyl allylamine;Allylbis(trimethylsilyl)amine;N,N'-bis(trimethylsilyl)allylamine;N-ALLYL-N N-BIS(TRIMETHYLSILYL)AMINE;Prop-2-en-1-ylbis(trimethylsilyl)amine;N-Allyl-N,N-bis(trimethylsilyl)amine 97%;2-ALLYL-1,1,1,3,3,3-HEXAMETHYLDISILAZAN&;N,N-bis(trimethylsilyl)prop-2-en-1-amine;N-allyl-1,1,1-trimethyl-N-(trimethylsilyl)silylamine
- CBNumber:
- CB4498643
- Molecular Formula:
- C9H23NSi2
- Molecular Weight:
- 201.46
- MOL File:
- 7688-51-9.mol
- MSDS File:
- SDS
- Modify Date:
- 2023/4/23 13:52:06
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS02,GHS07 |
|---|---|
| Signal word | Warning |
| Hazard statements | H226-H315-H319-H335 |
| Precautionary statements | P210-P302+P352-P305+P351+P338 |
| Hazard Codes | Xi |
| Risk Statements | 10-36/37/38 |
| Safety Statements | 16-26-36/37 |
| RIDADR | UN 1993 3/PG 3 |
| WGK Germany | 3 |
N-ALLYL-N N-BIS(TRIMETHYLSILYL)AMINE Chemical Properties,Uses,Production
Uses
N-Allyl-N,N-bis(trimethylsilyl)amine or N,N-bis(trimethylsilyl)prop-2-en-1-amine (AHMDS) can be used as a nucleophilic reagent to prepare:
- Complex bicyclic scaffolds by reacting with aryl aldehydes via imine formation followed by efficient multicomponent reactions.
- Dithiasuccinoyl (Dts) heterocycle by reacting with bisthiocarbamoyl chloride.
- Allyl selenide by reacting with aryl selenium salt in the presence of a ruthenium catalyst; Allyl selenide is utilized as a key intermediate in organic synthesis.
AHMDS can also be used as a reagent in hydrosilylation and hydroboration reaction. It may be also used as an electrolyte additive.
N-ALLYL-N N-BIS(TRIMETHYLSILYL)AMINE Preparation Products And Raw materials
Global( 50)Suppliers
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Shanghai Daken Advanced Materials Co.,Ltd | +86-371-66670886 | China | 16209 | 58 | Inquiry |
| Neostar United (Changzhou) Industrial Co., Ltd. | +86-519-519-85557386 | China | 12210 | 58 | Inquiry |
| Career Henan Chemica Co | +86-0371-86658258 +8613203830695 | China | 30253 | 58 | Inquiry |
| Hefei TNJ Chemical Industry Co.,Ltd. | 0551-65418671 | China | 34571 | 58 | Inquiry |
| CD Chemical Group Limited | +8615986615575 | China | 20356 | 58 | Inquiry |
| Zhengzhou Gecko Scientific Inc. | +8613938510057 | China | 283 | 58 | Inquiry |
| Hu Bei Jiutian Bio-medical Technology CO.,Ltd | 027-88013699 17354350817 | China | 7432 | 58 | Inquiry |
| ShenZhen Trendseen Biological Technology Co.,Ltd. | 13417589054 | China | 11681 | 58 | Inquiry |
| Aladdin Scientific | +1-+1(833)-552-7181 | United States | 57511 | 58 | Inquiry |
| Shanghai Aladdin Biochemical Technology Co.,Ltd. | +86-18521732826 | China | 48467 | 58 | Inquiry |
7688-51-9(N-ALLYL-N N-BIS(TRIMETHYLSILYL)AMINE)Related Search:
1,1,1-Trimethyl-N-2-propenyl-N-(trimethylsilyl)silanamine
Allylbis(trimethylsilyl)amine
α,α,α-Trimethyl-N-(2-propenyl)-N-(trimethylsilyl)silanamine
Einecs 231-699-2
N-ALLYL-N N-BIS(TRIMETHYLSILYL)AMINE
N-allyl-1,1,1-trimethyl-N-(trimethylsilyl)silylamine
2-ALLYL-1,1,1,3,3,3-HEXAMETHYLDISILAZAN&
N,N'-bis(trimethylsilyl)allylamine
Prop-2-en-1-ylbis(trimethylsilyl)amine
Silanamine, 1,1,1-trimethyl-N-2-propen-1-yl-N-(trimethylsilyl)-
N-Allyl-N,N-bis(trimethylsilyl)amine 97%
N,N-bis(trimethylsilyl)prop-2-en-1-amine
N-Allyl-1,1,1-trimethyl-N-(trimethylsilyl)silanamine
Ditrimethylsilyl allylamine
7688-51-9
CH2CHCH2NHSiCH332
C9H23NSi2
Organometallic Reagents
Organosilicon
Others





