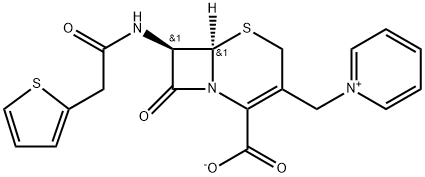
Cephaloridine
- CAS No.
- 50-59-9
- Chemical Name:
- Cephaloridine
- Synonyms
- Sefacin;Ceporin;Ceporan;Ceflorin;Keflodin;Keflordin;Sch 11527;Cephaloridine;Cephaloridine II;Cephaloridine USP/EP/BP
- CBNumber:
- CB4875354
- Molecular Formula:
- C19H17N3O4S2
- Molecular Weight:
- 415.48
- MOL File:
- 50-59-9.mol
- MSDS File:
- SDS
- Modify Date:
- 2024/8/28 13:53:23
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|---|---|
| Signal word | Warning |
| Hazard statements | H317 |
| Precautionary statements | P261-P272-P280-P302+P352-P333+P313-P321-P363-P501 |
| Hazard Codes | Xn |
| Risk Statements | 42/43 |
| Safety Statements | 22-36/37-45 |
| Hazardous Substances Data | 50-59-9(Hazardous Substances Data) |
| Toxicity | LD50 mice, rats (g/kg): >15, 2.5-4 orally; in monkeys (g/kg): >0.2 i.m. (Atkinson) |
Cephaloridine Chemical Properties,Uses,Production
Uses
Antibacterial agent.
Definition
ChEBI: A cephalosporin compound having pyridinium-1-ylmethyl and 2-thienylacetamido side-groups. A first-generation semisynthetic derivative of cephalosporin C.
World Health Organization (WHO)
Cefaloridine, a semi-synthetic cephalosporin antibiotic, was introduced into medicine in 1964 for the treatment of bacterial infections. It is considered to be the most toxic of the cephalosporins, and for this reason is now seldom used. Nevertheless, it still remains available in certain countries and the World Health Organization is not aware of restrictive actions taken elsewhere.
General Description
Cephaloridine, along with cephalothin, was the first member of the synthetic cephalosporin C class of antibiotic, to be introduced clinically. It was synthesized, starting with 7-aminocephalosporanic acid, by Glaxo Research Laboratories in 1962. This drug shows strong activity against gram-positive and gramnegative bacteria and Leptospira, including benzylpenicillin-resistant strains. It has been given widely by intravenous, intramuscular, and intraspinal injection to treat a variety of infections caused by Staphylococcus, Streptococcus, Neisseria, Klebsiella, Escherichia coli, and other pathogens. Because of its renal toxicity and the development of newer and more active synthetic cephalosporins, its use is declining.
Hazard
Moderately to very toxic.
Cephaloridine Preparation Products And Raw materials
Raw materials
Preparation Products
Cephaloridine Suppliers
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Pharmaffiliates Analytics and Synthetics P. Ltd | +91-172-5066494 | Haryana, India | 6739 | 58 | Inquiry |
| Central Drug House(P) Ltd. | 91-11-49404040 | New Delhi, India | 6157 | 58 | Inquiry |
| Pharma Affiliates | 172-5066494 | Haryana, India | 6754 | 58 | Inquiry |
| TargetMol Chemicals Inc. | +1-781-999-5354 +1-00000000000 | United States | 32159 | 58 | Inquiry |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418671 +8618949823763 | China | 34563 | 58 | Inquiry |
| Dideu Industries Group Limited | +86-29-89586680 +86-15129568250 | China | 22793 | 58 | Inquiry |
| AFINE CHEMICALS LIMITED | +86-0571-85134551 | China | 15350 | 58 | Inquiry |
| Dayang Chem (Hangzhou) Co.,Ltd. | 571-88938639 +8617705817739 | China | 52849 | 58 | Inquiry |
| GIHI CHEMICALS CO.,LIMITED | +8618058761490 | China | 49984 | 58 | Inquiry |
| Zhejiang Huida Biotech Co., LTD | 0571-0571-89903882 15990081639 | China | 3705 | 58 | Inquiry |
50-59-9(Cephaloridine)Related Search:
1of4
chevron_right




