SAMARIUM(II) IODIDE
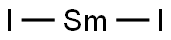
- CAS No.
- 32248-43-4
- Chemical Name:
- SAMARIUM(II) IODIDE
- Synonyms
- SAMARIUM IODIDE;Samarium iodide (SmI2);Diiodosamarium(II);SAMARIUM(II) IODIDE;Samarium(ll) iodide;0.1MinTHF,stabilized;Samarium(II) diiodide;Samarium(Ii)Iodide,0.1;SaMariuM(II) Iodide samarium(ii) iodide solution
- CBNumber:
- CB5168664
- Molecular Formula:
- I2Sm
- Molecular Weight:
- 404.17
- MOL File:
- 32248-43-4.mol
- MSDS File:
- SDS
- Modify Date:
- 2024/5/31 13:29:57
| Melting point | 526 ±3° |
|---|---|
| Boiling point | solid: 1580℃ [CRC10] |
| Density | 0.922 g/mL at 25 °C |
| Flash point | −2 °F |
| storage temp. | 2-8°C |
| solubility | reacts with H2O |
| form | powder |
| color | Deep blue-green |
| Sensitive | Air & Moisture Sensitive |
| Merck | 14,8349 |
| Exposure limits |
ACGIH: TWA 50 ppm; STEL 100 ppm (Skin) OSHA: TWA 200 ppm(590 mg/m3) NIOSH: IDLH 2000 ppm; TWA 200 ppm(590 mg/m3); STEL 250 ppm(735 mg/m3) |
| InChIKey | UAWABSHMGXMCRK-UHFFFAOYSA-L |
| CAS DataBase Reference | 32248-43-4(CAS DataBase Reference) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |     GHS02,GHS07,GHS08,GHS05 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H302-H315-H371-H372-H260-H314-H318-H225-H319-H335-H351-H373 | |||||||||
| Precautionary statements | P210-P260-P280-P305+P351+P338-P370+P378-P403+P235-P233-P240-P241+P242+P243-P264-P270-P271-P301+P312+P330-P302+P352+P332+P313+P362+P364-P304+P340+P312-P305+P351+P338+P337+P313-P308+P311-P403+P233-P405-P501-P223-P303+P361+P353-P501a | |||||||||
| Hazard Codes | F,Xi,Xn | |||||||||
| Risk Statements | 11-19-36/37-40 | |||||||||
| Safety Statements | 16-29-33-36/37-26 | |||||||||
| RIDADR | UN 2056 3/PG 2 | |||||||||
| WGK Germany | 3 | |||||||||
| F | 1-10 | |||||||||
| HazardClass | 3 | |||||||||
| PackingGroup | II | |||||||||
| HS Code | 28276000 | |||||||||
| NFPA 704 |
|
SAMARIUM(II) IODIDE price More Price(11)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | 347116 | Samarium(II) iodide solution 0.1?M in THF, contains samarium chips as stabilizer | 32248-43-4 | 4X25ML | ₹6841.4 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 409340 | Samarium(II) iodide anhydrous, powder, ≥99.9% trace metals basis | 32248-43-4 | 1G | ₹18445.8 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 409340 | Samarium(II) iodide anhydrous, powder, ≥99.9% trace metals basis | 32248-43-4 | 5G | ₹34347.73 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 347116 | Samarium(II) iodide solution 0.1?M in THF, contains samarium chips as stabilizer | 32248-43-4 | 25ML | ₹7036.25 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 347116 | Samarium(II) iodide solution 0.1?M in THF, contains samarium chips as stabilizer | 32248-43-4 | 100ML | ₹8086.28 | 2022-06-14 | Buy |
SAMARIUM(II) IODIDE Chemical Properties,Uses,Production
Description
Samarium(II) iodide, SmI2, when employed as a solution for organic synthesis, is known as “Kagan’s reagent.” SmI2 is a green solid and its solutions are green as well. It is a strong one-electron reducing agent that is used in organic synthesis. Solid, solvent-free SmI2 forms by high-temperature decomposition of samarium(III) iodide (SmI3).
Chemical Properties
Deep bleu-green solution
Uses
Samarium(II) iodide solution (SmI2) can be used as a reagent in the synthesis of:
- Benzannulated pyrrolizidines and indolizidines by SmI2-induced cyclizations of indole derivatives.
- Chiral 4-substituted 2-oxazolidinones and 5,5-disubstituted oxazolidinones through asymmetric Reformatsky type reaction.
- γ-Aminoalkyl substituted γ-butyrolactones via ketyl-alkene coupling reaction.
It can also be used in:
- Reduction of conjugated double and triple bonds into alkenes using SmI2/H2O/amine mixtures.
- Conversion of β-hydroxyketones into 1,3-diols by SmI2/H2O/Et3N.
- Selective reduction of 6-membered lactones to the corresponding diols/triols using SmI2-H2O reagent system.
SmI2 is an effective single-electron reducing agent for the promotion of ketone-olefin, ketyl aryl cyclizations, and pinacol coupling reactions under mild conditions. Often both intramolecular and intermolecular couplings proceed in a highly stereoselective fashion. It is also used in the synthesis of new heteroleptic samarium aryloxide/cyclopentadienide complexes.
Purification Methods
A possible impurity is SmI3 from which it is made. If present, grind the solid to a powder and heat it in a stream of pure H2. The temperature (~ 500-600o) should be below the m (~ 628o) of SmI3, since the molten compounds react very slowly. [Wetzel in Handbook of Preparative Inorganic Chemistry (Ed. Brauer) Academic Press Vol II pp 1149, 1150 1965.]
SAMARIUM(II) IODIDE Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| CLEARSYNTH LABS LTD. | +91-22-45045900 | Hyderabad, India | 6351 | 58 | Inquiry |
| CHEMSWORTH | +91-261-2397244 | New Delhi, India | 6707 | 30 | Inquiry |
| Otto Chemie Pvt. Ltd. | +91 9820041841 | Mumbai, India | 5873 | 58 | Inquiry |
| TCI Chemicals (India) Pvt. Ltd. | 1800 425 7889 | New Delhi, India | 6778 | 58 | Inquiry |
| Alfa Aesar | 1 800 209 7001 | Maharashtra, India | 6913 | 58 | Inquiry |
| Clearsynth Labs | 91-22-45045900 | Maharashtra, India | 3889 | 58 | Inquiry |
| Grr Fine Chem/Grr Exports | 91-79-40031771 | Gujarat, India | 405 | 58 | Inquiry |
| career henan chemical co | +86-0371-86658258 +8613203830695 | China | 29899 | 58 | Inquiry |
| Chongqing Chemdad Co., Ltd | +86-023-6139-8061 +86-86-13650506873 | China | 39916 | 58 | Inquiry |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | China | 49391 | 58 | Inquiry |
| Supplier | Advantage |
|---|---|
| CLEARSYNTH LABS LTD. | 58 |
| CHEMSWORTH | 30 |
| Otto Chemie Pvt. Ltd. | 58 |
| TCI Chemicals (India) Pvt. Ltd. | 58 |
| Alfa Aesar | 58 |
| Clearsynth Labs | 58 |
| Grr Fine Chem/Grr Exports | 58 |
| career henan chemical co | 58 |
| Chongqing Chemdad Co., Ltd | 58 |
| CONIER CHEM AND PHARMA LIMITED | 58 |
32248-43-4(SAMARIUM(II) IODIDE)Related Search:
1of4
chevron_right




