Sodium iodide
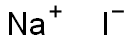
- CAS No.
- 7681-82-5
- Chemical Name:
- Sodium iodide
- Synonyms
- Sodium iodine;Anayodin;ioduredesodium;Sodium iodide (NaI);Hydriodic acid sodium salt;Ioduril;Soiodin;jodidsodny;Jodid sodny;Natriumjodid
- CBNumber:
- CB6170714
- Molecular Formula:
- NaI
- Molecular Weight:
- 149.89
- MOL File:
- 7681-82-5.mol
- MSDS File:
- SDS
- Modify Date:
- 2025/1/27 9:38:02
| Melting point | 661 °C (lit.) |
|---|---|
| Boiling point | 1300 °C |
| Density | 3.66 |
| bulk density | 1500-2000kg/m3 |
| vapor density | >1 (vs air) |
| vapor pressure | 1 hPa (767 °C) |
| refractive index | 1.7745 |
| Flash point | 1300-1304°C |
| storage temp. | Store at +5°C to +30°C. |
| solubility | water: soluble(lit.) |
| form | beads |
| pka | 0.067[at 20 ℃] |
| Specific Gravity | 3.67 |
| color | White |
| PH | 6-9 (50g/l, H2O, 20℃) |
| Water Solubility | 184 g/100 mL (25 ºC) |
| Sensitive | Hygroscopic |
| Merck | 14,8631 |
| Exposure limits | ACGIH: TWA 0.01 ppm |
| Dielectric constant | 7.2800000000000002 |
| Stability | hygroscopic |
| InChIKey | FVAUCKIRQBBSSJ-UHFFFAOYSA-M |
| LogP | -1.301 at 25℃ |
| CAS DataBase Reference | 7681-82-5(CAS DataBase Reference) |
| NIST Chemistry Reference | Sodium iodide(7681-82-5) |
| EPA Substance Registry System | Sodium iodide (7681-82-5) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS07,GHS08,GHS09 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H315-H319-H372-H400 | |||||||||
| Precautionary statements | P260-P264-P273-P302+P352-P305+P351+P338-P314 | |||||||||
| Hazard Codes | Xi,N | |||||||||
| Risk Statements | 36/38-36/37/38-50 | |||||||||
| Safety Statements | 26-61 | |||||||||
| RIDADR | UN 3077 9 / PGIII | |||||||||
| WGK Germany | 1 | |||||||||
| RTECS | WB6475000 | |||||||||
| F | 8 | |||||||||
| TSCA | Yes | |||||||||
| HS Code | 28276000 | |||||||||
| Hazardous Substances Data | 7681-82-5(Hazardous Substances Data) | |||||||||
| Toxicity | MLD i.v. in rats: 1.3 g/kg, Loeser, Konwiser, J. Lab. Clin. Med. 15, 35 (1929) | |||||||||
| NFPA 704 |
|
Sodium iodide price More Price(60)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | 793558 | Sodium iodide anhydrous, free-flowing, Redi-Dri?, ReagentPlus?, ≥99% | 7681-82-5 | 100G | ₹3409.88 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 746371 | Sodium iodide anhydrous, free-flowing, Redi-Dri?, ACS reagent, ≥99.5% | 7681-82-5 | 100G | ₹5055.28 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 793558 | Sodium iodide anhydrous, free-flowing, Redi-Dri?, ReagentPlus?, ≥99% | 7681-82-5 | 500G | ₹7555.85 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 793558 | Sodium iodide anhydrous, free-flowing, Redi-Dri?, ReagentPlus?, ≥99% | 7681-82-5 | 1KG | ₹14906.03 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 746371 | Sodium iodide anhydrous, free-flowing, Redi-Dri?, ACS reagent, ≥99.5% | 7681-82-5 | 500G | ₹28036.75 | 2022-06-14 | Buy |
Sodium iodide Chemical Properties,Uses,Production
Chemical Properties
White or almost white, crystalline powder or colourless crystals, hygroscopic.
Physical properties
Physical Properties White crystalline deliquescent powder or granules; saline and slight bitter taste; absorbs moisture from air; slowly turns brown on exposure to air due to iodine evolved; density 3.67g/cm3; melts at 660°C; vaporizes at 1,304°C; vapor pressure 1 torr at 767°C and 5 torr at 857°C; very soluble in water, 178.7 g/100 mL at 20°C and 294 g/100 mL at 70°C; soluble in ethanol and acetone.
Uses
Sodium iodide is widely used for halide exchange (Finkelstein reaction), for example in the conversion of an alkyl chloride, allyl chloride and arylmethyl chloride into their respective iodides, which are precursors for pharmaceutical and fine chemical products. They are used to ehnance the efficiency of the formation of Wittig adducts from less reactive chlorides and bromides. Appropriate prepartions find use as a nutrient supplement. Sodium iodide is used as the precursor to the control agent in ab initio emulsion polymerization. Sodium iodide finds use in the determination of dissolved oxygen in the modified Winkler method, synthesis of the fluorescent dye coppersensor-1 (CS1) for imaging labile copper pools in biological samples, and the cleavage of esters, lactones, carbamates and ethers in combination with chlorotrimethylsilane.
Preparation
Sodium iodide is prepared by adding hydriodic acid or an acidic iodide solution to a solution of sodium hydroxide or sodium carbonate, followed by evaporation and crystallization: NaOH + HI → NaI + H2O
The solution is filtered to remove any impurities prior to its evaporation and crystallization.
Definition
sodium iodide: A white crystallinesolid, NaI, very soluble in water andsoluble in both ethanol and ethanoicacid. It is known in both the anhydrousform (cubic; r.d. 3.67; m.p.661°C; b.p. 1304°C) and as the dihydrate(monoclinic; r.d. 2.45). It is preparedby the reaction of hydrogeniodide with sodium carbonate orsodium hydroxide in solution. Likepotassium iodide, sodium iodide inaqueous solution dissolves iodine toform a brown solution containingthe I3- ion. It finds applications inphotography and is also used in medicineas an expectorant and in the administrationof radioactive iodine for studies of thyroid function and fortreatment of diseases of the thyroid.
General Description
Sodium iodide is an inorganic salt. Mixture of chlorotrimethylsilane/sodium iodide in acetonitrile is a useful reagent for the cleavage of esters, lactones, carbamates and ethers.
Safety Profile
Moderately toxic by ingestion, intravenous, and intraperitoneal routes. Human teratogenic effects by ingestion: developmental abnormalities of the endocrine system. Human reproductive effects by ingestion: effects on newborn, including postnatal measurements. A skin and eye irritant. Reacts violently with BrF3, HClO4, oxidants. When heated to decomposition it emits toxic fumes of Iand Na2O. See also IODIDES.
Purification Methods
Crystallise NaI from water/ethanol solution and dry it for 12hours under vacuum, at 70o. Alternatively, dissolve it in acetone, filter it and cool it to -20o; the resulting yellow crystals are filtered off and heated in a vacuum oven at 70o for 6hours to remove acetone. The NaI is then crystallised from very dilute NaOH, dried under vacuum, and stored in a vacuum desiccator [Verdin Trans Faraday Soc 57 484 1961].
Sodium iodide Preparation Products And Raw materials
Raw materials
Preparation Products
1of8
chevron_right| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| SAGAR LIFE SCIENCES PVT LTD | +91-8770359041 +91-8770359041 | Gujarat, India | 79 | 58 | Inquiry |
| SALVI CHEMICAL | +91-9321313096 +91-9321313096 | Mumbai, India | 26 | 58 | Inquiry |
| Calibre Chemicals Pvt. Limited | +91-9930991722 +91-9930991722 | Gujarat, India | 8 | 58 | Inquiry |
| Dynarx Technology India Limited | +91-9960099480 +91-9960099480 | Bangalore, India | 71 | 58 | Inquiry |
| Eskay Iodine Pvt Ltd | +91-22-6622 7575 | Mumbai, India | 28 | 58 | Inquiry |
| SL Drugs & Pharmaceuticals | +91-4023840292 +91-4023840292 | Hyderabad, India | 12 | 58 | Inquiry |
| Laksh Finechem Pvt.Ltd. | +91-9978522233 +91-78522233 | Gujarat, India | 55 | 58 | Inquiry |
| Infinium Pharmachem Limited | +91-2692238849 +91-2692238850 | Gujarat, India | 65 | 58 | Inquiry |
| Yogi Dye Chem Industries | +91-9821098577 +91-9821098577 | Mumbai, India | 124 | 58 | Inquiry |
| Shreenath Chemicals | +91-8369634120 +91-8369634120 | Maharashtra, India | 48 | 58 | Inquiry |
Related articles
- Sodium iodide: Chemical property, Benefits, and Pharmacokinetic
- Sodium iodide is a water-soluble ionic compound with a crystal lattice. Sodium iodide is a source of iodine and can be adminis....
- Sep 5,2024
- Sodium Iodide Crystal
- The space lattice of Sodium iodide(NaI) belongs to the cubic system, and its rock salt structure has a lattice constant of a=0....
- Nov 20,2023
7681-82-5(Sodium iodide )Related Search:
1of4
chevron_right




