Sodium hydroxide
- CAS No.
- 1310-73-2
- Chemical Name:
- Sodium hydroxide
- Synonyms
- NaOH;Caustic soda;Caustic Soda flakes;Caustic soda pearl;CAUSTIC FLAKES;Natriumhydroxid;IODINE SOLUTION;FLAKE CAUSTIC SODA;Hydroxyde de sodium;SODIUM HYDROXIDE, REAGENT GRADE, 97%, FL
- CBNumber:
- CB8105015
- Molecular Formula:
- NaOH
- Molecular Weight:
- 39.99711
- MOL File:
- 1310-73-2.mol
- MSDS File:
- SDS
- Modify Date:
- 2024/6/22 7:31:40
| Melting point | 681 °C(lit.) |
|---|---|
| Boiling point | 1390°C |
| Density | 1.515 g/mL at 20 °C |
| vapor density | <1 (vs air) |
| vapor pressure | 1 mm Hg ( 745 °C) |
| refractive index | 1,473-1,475 |
| Flash point | 176-178°C |
| storage temp. | room temp |
| solubility | H2O: 1 M at 20 °C, clear, colorless |
| form | beads |
| Specific Gravity | 2.13 |
| color | White |
| Odor | Odorless |
| PH Range | 13 - 14 |
| PH | 10.98(1 mM solution);11.95(10 mM solution);12.88(100 mM solution); |
| Water Solubility | SOLUBLE |
| Sensitive | Air Sensitive & Hygroscopic |
| Decomposition | 176-178 ºC |
| λmax |
λ: 260 nm Amax: 0.015 λ: 280 nm Amax: 0.01 |
| Merck | 14,8627 |
| Exposure limits | TLV-TWA air 2 mg/m3 (OSHA); ceiling 2 mg/m3 (ACGIH) and 2 mg/m3/15 min (NIOSH). |
| Dielectric constant | 57.5(25℃) |
| Stability | hygroscopic |
| CAS DataBase Reference | 1310-73-2(CAS DataBase Reference) |
| NIST Chemistry Reference | Sodium hydroxide(1310-73-2) |
| EPA Substance Registry System | Sodium hydroxide (1310-73-2) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS05 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H290-H314 | |||||||||
| Precautionary statements | P234-P260-P280-P303+P361+P353-P304+P340+P310-P305+P351+P338 | |||||||||
| Hazard Codes | C,Xi | |||||||||
| Risk Statements | 36/38-35-34 | |||||||||
| Safety Statements | 26-45-37/39-24/25-36/37/39 | |||||||||
| OEL | Ceiling: 2 mg/m3 | |||||||||
| RIDADR | UN 1824 8/PG 2 | |||||||||
| WGK Germany | 1 | |||||||||
| RTECS | TT2975000 | |||||||||
| F | 8 | |||||||||
| TSCA | Yes | |||||||||
| HS Code | 2815 11 00 | |||||||||
| HazardClass | 8 | |||||||||
| PackingGroup | II | |||||||||
| Toxicity | LD orally in rabbits: 500 mg/kg (10% soln) (Fazekas) | |||||||||
| IDLA | 10 mg/m3 | |||||||||
| NFPA 704 |
|
Sodium hydroxide price More Price(58)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | 223921 | Ascarite® Sodium hydroxide-coated silica, 20-30?mesh | 1310-73-2 | 100G | ₹14224.05 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 223921 | Ascarite® Sodium hydroxide-coated silica, 20-30?mesh | 1310-73-2 | 500G | ₹62709.23 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 223913 | Ascarite® Sodium hydroxide-coated silica, 8-20?mesh | 1310-73-2 | 100G | ₹19052 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 223913 | Ascarite® Sodium hydroxide-coated silica, 8-20?mesh | 1310-73-2 | 500G | ₹48084.65 | 2022-06-14 | Buy |
| TCI Chemicals (India) | S0543 | Sodium Hydroxide (2mol/L in Water) | 1310-73-2 | 500ML | ₹1900 | 2022-05-26 | Buy |
Sodium hydroxide Chemical Properties,Uses,Production
Chemical Properties
Sodium hydroxide, NaOH,also referred to as caustic soda or sodium hydrate(and formerly known as lye), is a white,massive, deliquescent crystalline solid that is soluble in water,alcohol, and glycerol. It melts at 318°C (606 OF) and is the most widely used and available alkaline chemical. Most sodium hydroxide is produced as a coproduct of chlorine through the use of electrolytic cells;the cells are of the diaphragm, mercury, or membrane type. Some sodium hydroxide is marked as produced in the cells;most is evaporated and sold as 50% and 73% solutions or as anhydrous beads. Most caustic end uses require solutions of relatively low concentrations. Caustic soda is used as an analytical reagent and chemical intermediate, in scouring and cleaning baths,in rubber reclaiming and petroleum refining, in quenching baths for heat treating of steel,in cutting and soluble oils,in soaps and detergents, and in a wide variety of other applications.
Physical properties
White orthorhombic crystals, produced in the form of pellets, lumps, sticks, beads, chips, flakes or solutions; hygroscopic; very corrosive; rapidly absorbs CO2 and water from the air; density 2.13 g/cm3; melts at 323°C; vaporizes at 1388°C; vapor pressure 1 torr at 739°C and 5 torr at 843°C; very soluble in water (110 g/100mL at room temperature), generating heat on dissolution; aqueous solutions highly alkaline, pH of 0.5% solution about 13 and 0.05% solution about 12; soluble in methanol, ethanol and glycerol (23.8 g/100 mL methanol and 13.9 g/100 mL ethanol at ambient temperatures.).
Uses
Sodium hydroxide is one of the most important industrial chemicals. In volume, it is in the top ten chemicals produced in the United States. It is used in manufacturing a large number of compounds including several sodium salts, in treating cellulose for producing rayon and cellophane, and in manufacturing soaps, detergents, pulp, and paper. Sodium hydroxide is a common neutralizing agent for acids in acid-base titrations and petroleum refining. Another major application is extracting metals from their ores where alkali fusion, such as fusion with caustic soda, often is applied to open the ores. Additionally, sodium hydroxide is used to precipitate metals as hydroxides. Other uses are in reclaiming rubber, dissolving casein in plastics production, refining vegetable oils, processing textiles, as an eluant in ion chromatography, etching and electroplating, and as a laboratory reagent. Sodium hydroxide also is used as a strong base in many organic synthesis and base-catalyzed reactions.
Production Methods
Sodium hydroxide is manufactured by electrolysis of brine using
inert electrodes. Chlorine is evolved as a gas at the anode and
hydrogen is evolved as a gas at the cathode. The removal of chloride
and hydrogen ions leaves sodium and hydroxide ions in solution.
The solution is dried to produce the solid sodium hydroxide.
A second method uses the Kellner–Solvay cell. Saturated sodium
chloride solution is electrolyzed between a carbon anode and a
flowing mercury cathode. In this case the sodium is produced at the
cathode rather than the hydrogen because of the readiness of
sodium to dissolve in the mercury. The sodium–mercury amalgam is
then exposed to water and a sodium hydroxide solution is
produced.
Definition
The most important commercial caustic.
Reactions
Sodium hydroxide is strongly alkaline and can react with acids to form salts and water.
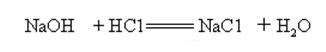
Sodium hydroxide reacts with acidic oxides to form salt and water, so sodium hydroxide can be used to absorb acid gases in the laboratory or industrially.
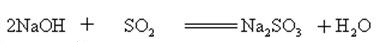
Sodium hydroxide can react with aqueous solutions of many metal salts to form sodium salts and metal hydroxides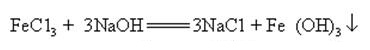
When sodium hydroxide and ammonia salt are heated together, it can release ammonia 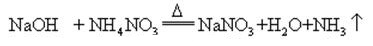
Sodium hydroxide is highly corrosive, so that the glass bottles storing sodium hydroxide solutions must be rubber stoppers, and glass stoppers should not be used to prevent a chemical reaction from opening. Sodium hydroxide is an important industrial raw material, and can be produced by electrolysis of saline solution industrially
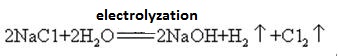
General Description
A white solid. Corrosive to metals and tissue. Used in chemical manufacturing, petroleum refining, cleaning compounds, drain cleaners.
Air & Water Reactions
Soluble in water. Dissolution can liberate enough heat to cause steaming and spattering and ignite adjacent combustible material [Haz. Chem. Data 1966].
Hazard
Corrosive to tissue in presence of mois- ture, strong irritant to tissue (eyes, skin, mucous membranes, and upper respiratory tract), poison by ingestion.
Health Hazard
Sodium hydroxide is a highly corrosive substancethat causes damage to human tissues.Its action on the skin is somewhat differentfrom acid burns. There is no immediate pain,but it penetrates the skin. It does not coagulateprotein to prevent its further penetration,and thus the caustic burn can become severeand slow healing. Spilling of its concentratedsolutions into the eyes can result in severeirritation or permanent injury.
It is toxic by ingestion as well as inhalationof its dust. Although the oral toxicity ofa 5–10% solution of caustic soda was foundto be low in test animals, high dosages atgreater concentrations can cause vomiting,prostration, and collapse. The oral lethal dosein rabbits is 500 mg/kg (NIOSH 1986).
Sodium hydroxide dusts or aerosols areirritating to the eyes, nose, and throat. Prolongedexposure to high concentrations in airmay produce ulceration of the nasal passage.
Fire Hazard
Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Some are oxidizers and may ignite combustibles (wood, paper, oil, clothing, etc.). Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated.
Flammability and Explosibility
Sodium hydroxide and potassium hydroxide are not flammable as solids or aqueous solutions.
Pharmaceutical Applications
Sodium hydroxide is widely used in pharmaceutical formulations to adjust the pH of solutions. It can also be used to react with weak acids to form salts.
Industrial uses
Caustic soda (NaOH) is regarded as the strongest alkaline pH regulator. Caustic soda
is a very active substance and is highly corrosive. The bulk of caustic soda is manufactured
by electrolysis of saturated brines (NaCl). Caustic soda has a very strong pHregulating
capability (i.e. from pH 7 to pH 14) at a relatively low dosage compared to
other alkaline substances. Commercially, caustic soda is available in anhydrous form,
but in most mining applications the caustic soda is supplied as a 50% solution.
In the mineral processing industry, sodium hydroxide is mostly used for alkalinity control
during the processing of non-metallic minerals. In base metal flotation, the use of
sodium hydroxide is rare.
Safety Profile
Poison by intraperitoneal route. Moderately toxic by ingestion. Mutation data reported. A corrosive irritant to skin, eyes, and mucous membranes. When heated to decomposition it emits toxic fumes of NanO.
Safety
Sodium hydroxide is widely used in the pharmaceutical and food
industries and is generally regarded as a nontoxic material at low
concentrations. At high concentrations it is a corrosive irritant to
the skin, eyes, and mucous membranes.
LD50 (mouse, IP): 0.04 g/kg
LD50 (rabbit, oral): 0.5 g/kg
Potential Exposure
NaOH is utilized to neutralize acids and make sodium salts in petroleum refining, viscose rayon; cellophane, plastic production; and in the reclamation of solutions of their salts. It is used in the manufacture of mercerized cotton, paper, explosives, and dyestuffs in metal cleaning; electrolytic extraction of zinc; tin plating; oxide coating; laundering, bleaching, dishwashing; and it is used in the chemical industries.
storage
Sodium hydroxide should be stored in an airtight nonmetallic container in a cool, dry place. When exposed to air, sodium hydroxide rapidly absorbs moisture and liquefies, but subsequently becomes solid again owing to absorption of carbon dioxide and formation of sodium carbonate.
Shipping
UN1823 NaOH, solid, Hazard class: 8; Labels: 8-Corrosive material. UN1824 NaOH, solution, Hazard class: 8; Labels: 8-Corrosive material
Incompatibilities
Sodium hydroxide is a strong base and is incompatible with any compound that readily undergoes hydrolysis or oxidation. It will react with acids, esters, and ethers, especially in aqueous solution.
Waste Disposal
Discharge into tank containing water, neutralize, then flush to sewer with water.
Regulatory Status
GRAS listed. Accepted for use as a food additive in Europe. Included in the FDA Inactive Ingredients Database (dental preparations; injections; inhalations; nasal, ophthalmic, oral, otic, rectal, topical, and vaginal preparations). Included in nonparenteral and parenteral medicines licensed in the UK. Included in the Canadian List of Acceptable Non-medicinal Ingredients.
Sodium hydroxide Preparation Products And Raw materials
Raw materials
1of3
chevron_rightPreparation Products
1of8
chevron_right| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Otto Chemie Pvt. Ltd. | +91 9820041841 | Mumbai, India | 32 | 58 | Inquiry |
| ANJI BIOSCIENCES | +91-9000100077 +91-9000100077 | Hyderabad, India | 430 | 58 | Inquiry |
| SUKHA CHEMICAL INDUSTRIES | +91-9638875444 +91-9638875444 | Gujarat, India | 26 | 58 | Inquiry |
| Soham Chemical Industries | +91-7016081644 +91-7016081644 | Mumbai, India | 83 | 58 | Inquiry |
| SHIVAM INDUSTRIES | +91-9820823043 +91-9820823043 | Mumbai, India | 30 | 58 | Inquiry |
| oswal chemicals | +91-9173644055 +91-9173644055 | Gujarat, India | 66 | 58 | Inquiry |
| Nyne Organics Pvt Ltd | +91-9920180386 +91-9920180386 | Mumbai, India | 53 | 58 | Inquiry |
| Chem stride | +91-8169461298 +91-8169461298 | Maharashtra, India | 27 | 58 | Inquiry |
| UNILOSA INTERNATINAL PRIVATE LIMITED | +91-9999069917 +91-9999069917 | New Delhi, India | 152 | 58 | Inquiry |
| Choice Chemicals | +91-9895031268 +91-9895031268 | Kerela, India | 118 | 58 | Inquiry |
| Supplier | Advantage |
|---|---|
| Otto Chemie Pvt. Ltd. | 58 |
| ANJI BIOSCIENCES | 58 |
| SUKHA CHEMICAL INDUSTRIES | 58 |
| Soham Chemical Industries | 58 |
| SHIVAM INDUSTRIES | 58 |
| oswal chemicals | 58 |
| Nyne Organics Pvt Ltd | 58 |
| Chem stride | 58 |
| UNILOSA INTERNATINAL PRIVATE LIMITED | 58 |
| Choice Chemicals | 58 |
Related articles
- Sodium hydroxide:pH calculation,Uses,Health hazards
- Liquid sodium hydroxide is colorless and has no odor. It can react violently with strong acids and with water.
- Mar 6,2024
- Uses and safety of Sodium hydroxide
- Sodium hydroxide is sometimes called caustic soda or lye. It is a common ingrediet in cleaners and soaps.
- Jul 18,2022
- The review of sodium hydroxide
- Sodium hydroxide, also known as caustic soda, lye and caustic soda, with the chemical formula of NaOH, is a strong alkali with....
- Mar 17,2022
1310-73-2(Sodium hydroxide)Related Search:
1of4
chevron_right




