Lithium hydroxide
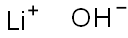
- CAS No.
- 1310-65-2
- Chemical Name:
- Lithium hydroxide
- Synonyms
- LiOH;ithium hydroxide;LithiuM hydroxide,anhydro;Lithium hydroxide ,99% [anhydrous];"Lithium hydroxide, anhydrous/ 99%";Lithium hydoxide;Lithium hydroxide;HEXANE, 95+%, PRA GRADE;lithiumhydroxide(li(oh));lithiumhydroxideanhydrous
- CBNumber:
- CB5406836
- Molecular Formula:
- LiOH
- Molecular Weight:
- 23.95
- MOL File:
- 1310-65-2.mol
- MSDS File:
- SDS
- Modify Date:
- 2024/5/7 14:24:42
| Melting point | 470 °C (dec.) (lit.) |
|---|---|
| Boiling point | 925°C |
| Density | 1.43 |
| storage temp. | Store below +30°C. |
| solubility | water: soluble71g/L at 20°C |
| form | Solid |
| pka | 14[at 20 ℃] |
| Specific Gravity | 2.54 |
| color | White to light yellow |
| Odor | odorless |
| PH | 12 (50g/l, H2O, 50℃) |
| Water Solubility | 113 g/L (20 ºC) |
| Sensitive | Air Sensitive & Hygroscopic |
| Merck | 14,5534 |
| Stability | Stable. Incompatible with moisture. strong acids, carbon dioxide. |
| InChIKey | WMFOQBRAJBCJND-UHFFFAOYSA-M |
| CAS DataBase Reference | 1310-65-2(CAS DataBase Reference) |
| NIST Chemistry Reference | Lithium hydroxide(1310-65-2) |
| EPA Substance Registry System | Lithium hydroxide (1310-65-2) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS05,GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H302-H314 | |||||||||
| Precautionary statements | P260-P270-P280-P301+P312-P303+P361+P353-P305+P351+P338 | |||||||||
| Hazard Codes | C | |||||||||
| Risk Statements | 52/53-35-20/22-22-34-23 | |||||||||
| Safety Statements | 45-36/37/39-26-22 | |||||||||
| RIDADR | 2680 | |||||||||
| WGK Germany | 1 | |||||||||
| RTECS | OJ6307070 | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 8 | |||||||||
| PackingGroup | II | |||||||||
| HS Code | 28252010 | |||||||||
| Toxicity | LD50 orally in Rabbit: 210 mg/kg | |||||||||
| NFPA 704 |
|
Lithium hydroxide price More Price(15)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | 545856 | Lithium hydroxide powder, reagent grade, ≥98% | 1310-65-2 | 100G | ₹2294.9 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 545856 | Lithium hydroxide powder, reagent grade, ≥98% | 1310-65-2 | 500G | ₹9428.58 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 442410 | Lithium hydroxide reagent grade, 98% | 1310-65-2 | 100G | ₹2836.15 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 442410 | Lithium hydroxide reagent grade, 98% | 1310-65-2 | 500G | ₹9428.58 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 1.05691 | Lithium hydroxide 98%+ for analysis EMSURE? | 1310-65-2 | 100G | ₹9180 | 2022-06-14 | Buy |
Lithium hydroxide Chemical Properties,Uses,Production
Chemical Properties
lithium hydroxide (LiOH) is a white solid made industrially as the monohydrate (LiOH.H2O) by reacting lime with a lithium ore or with a salt made from the ore. Lithium hydroxide has a closer resemblance to the group 2 hydroxides than to the group 1 hydroxides.
Physical properties
White tetragonal crystals; refractive index 1.464; density 1.46 g/cm3; melts at 450°C; decomposes at 924°C; dissolves in water (12.8g/100g at 20°C and 17.5 g/100g at 100°C); slightly soluble in alcohol. The monohydrate is white monoclinic crystalline solid; refractive index 1.460; density 1.51 g/cm3; soluble in water, more soluble than the anhydrous salt (22.3g and 26.8g/100g at 10 and 100°C, respectively); slightly soluble in alcohol; insoluble in ether.
Uses
Lithium hydroxide is used in storage batteries and soaps and as CO2 absorber in spacecrafts.
Definition
A white crystallinesolid, LiOH, soluble in water,slightly soluble in ethanol and insolublein ether. It is known as the monohydrate(monoclinic; r.d. 1.51) and inthe anhydrous form (tetragonal, r.d.1.46; m.p. 450°C; decomposes at924°C). The compound is made by reacting lime with lithium salts orlithium ores. Lithium hydroxide isbasic but has a closer resemblance togroup 2 hydroxides than to the othergroup 1 hydroxides (an example ofthe first member of a periodic grouphaving atypical properties).
General Description
A clear to water-white liquid which may have a pungent odor. Contact may cause severe irritation to skin, eyes, and mucous membranes. Lithium hydroxide may be toxic by ingestion, inhalation and skin absorption. Lithium hydroxide is used to make other chemicals.
Air & Water Reactions
Dilution with water may generate enough heat to cause steaming or spattering.
Reactivity Profile
LITHIUM HYDROXIDE SOLUTION neutralizes acids exothermically to form salts plus water. Reacts with certain metals (such as aluminum and zinc) to form oxides or hydroxides of the metal and generate gaseous hydrogen. May initiate polymerization reactions in polymerizable organic compounds, especially epoxides. May generate flammable and/or toxic gases with ammonium salts, nitrides, halogenated organics, various metals, peroxides, and hydroperoxides. May serve as a catalyst. Reacts when heated above about 84°C with aqueous solutions of reducing sugars other than sucrose, to evolve toxic levels of carbon monoxide [Bretherick, 5th Ed., 1995].
Health Hazard
TOXIC; inhalation, ingestion or skin contact with material may cause severe injury or death. Contact with molten substance may cause severe burns to skin and eyes. Avoid any skin contact. Effects of contact or inhalation may be delayed. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.
Fire Hazard
Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Some are oxidizers and may ignite combustibles (wood, paper, oil, clothing, etc.). Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated.
Safety Profile
Poison by ingestion and subcutaneous routes. Mtldly toxic by inhalation. A corrosive. When heated to decomposition it emits toxic fumes of Li.
Purification Methods
It crystallises from hot water (3mL/g) as the monohydrate. It is dehydrated at 150o in a stream of CO2-free air. It sublimes at 220o with partial decomposition [Cohen Inorg Synth V 3 1957, Bravo Inorg Synth VII 1 1963].
Lithium hydroxide Preparation Products And Raw materials
Raw materials
Preparation Products
1of5
chevron_right| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Agilenobel | +91-8090295395 +91-8800293647 | Haryana, India | 50 | 58 | Inquiry |
| Parad Corporation Pvt Ltd | +91-7433900100 +91-9426510550 | Gujarat, India | 37 | 58 | Inquiry |
| FMC India Private Limited | Karnataka, India | 16 | 58 | Inquiry | |
| TCI Chemicals (India) Pvt. Ltd. | 1800 425 7889 | New Delhi, India | 6778 | 58 | Inquiry |
| Saradhi Organics | 91-7660890360 | Hyderabad, India | 184 | 58 | Inquiry |
| Laurice Labs | 08048372498Ext 403 | Mumbai, India | 58 | 58 | Inquiry |
| Indian Platinum Pvt., Ltd. | 91-22-26162085 | Maharashtra, India | 66 | 58 | Inquiry |
| Chemico | 09166312452 | Mumbai, India | 235 | 58 | Inquiry |
| Satyan Pharmaceuticals Pvt Ltd. | 91-22-67330300 | Maharashtra, India | 54 | 58 | Inquiry |
| J. N. Chemical | 91-260-2435225 | Gujarat, India | 139 | 58 | Inquiry |
| Supplier | Advantage |
|---|---|
| Agilenobel | 58 |
| Parad Corporation Pvt Ltd | 58 |
| FMC India Private Limited | 58 |
| TCI Chemicals (India) Pvt. Ltd. | 58 |
| Saradhi Organics | 58 |
| Laurice Labs | 58 |
| Indian Platinum Pvt., Ltd. | 58 |
| Chemico | 58 |
| Satyan Pharmaceuticals Pvt Ltd. | 58 |
| J. N. Chemical | 58 |
Related articles
- Introduction of Lithium hydroxide
- Lithium hydroxide, solution appears as a clear to water-white liquid which may have a pungent odor. It is used to make other c....
- Feb 28,2022
1310-65-2(Lithium hydroxide)Related Search:
1of4
chevron_right




