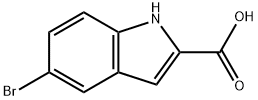
5-Bromoindole-2-carboxylic acid
- CAS No.
- 7254-19-5
- Chemical Name:
- 5-Bromoindole-2-carboxylic acid
- Synonyms
- 5-BROMO-1H-INDOLE-2-CARBOXYLIC ACID;NSC 73384;AKOS JY2082545;5-Bromo-2-carboxy-1H-indole;5-BROMOINDOLE-2-CARBOXYLIC ACID;5-BROMO-2-INDOLECARBOXYLIC ACID;5-Bromoindole-2-carboxylicAcid>5-Bromoindazole-2-carboxylic acid;5-BroMo-1H-indol-2-carboxylic acid;5-Bromoindole-2-carboxylic acid ,98%
- CBNumber:
- CB0242185
- Molecular Formula:
- C9H6BrNO2
- Molecular Weight:
- 240.05
- MOL File:
- 7254-19-5.mol
- MSDS File:
- SDS
- Modify Date:
- 2024/8/7 19:09:42
| Melting point | 287-288 |
|---|---|
| Boiling point | 470.9±25.0 °C(Predicted) |
| Density | 1.838±0.06 g/cm3(Predicted) |
| storage temp. | -20°C |
| pka | 4.25±0.30(Predicted) |
| form | Solid |
| color | White to Light yellow to Light orange |
| InChI | InChI=1S/C9H6BrNO2/c10-6-1-2-7-5(3-6)4-8(11-7)9(12)13/h1-4,11H,(H,12,13) |
| InChIKey | YAULOOYNCJDPPU-UHFFFAOYSA-N |
| SMILES | N1C2=C(C=C(Br)C=C2)C=C1C(O)=O |
| CAS DataBase Reference | 7254-19-5(CAS DataBase Reference) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H335-H315-H319 | |||||||||
| Precautionary statements | P264-P280-P302+P352+P332+P313+P362+P364-P305+P351+P338+P337+P313-P261-P280a-P304+P340-P305+P351+P338-P405-P501a | |||||||||
| Hazard Codes | Xi | |||||||||
| Risk Statements | 36/37/38 | |||||||||
| Safety Statements | 26-37/39-36/37/39 | |||||||||
| WGK Germany | 3 | |||||||||
| HazardClass | IRRITANT | |||||||||
| HS Code | 29339980 | |||||||||
| NFPA 704 |
|
5-Bromoindole-2-carboxylic acid price More Price(2)
5-Bromoindole-2-carboxylic acid Chemical Properties,Uses,Production
Uses
5-Bromoindole-2-carboxylic acid,Reactant involved in studies of biologically active molecules including:Discovery of indole inhibitors of MMP-13 for treatment of arthritic diseases1;Synthesis of indolyl ethanones as indoleamine 2,3-dioxygenase inhibitors2 ;cis-Diaminocyclohexane derivatives prepared for use as factor Xa inhibitors3 ;Synthesis of tubulin polymerization inhibitors and cancer cell growth inhibitors4 ;Preparation of dual PPARγ/δ agonists5;Synthesis of chemical probes to examine the role of hFPRL1 receptor .
5-Bromoindole-2-carboxylic acid Preparation Products And Raw materials
Global( 329)Suppliers
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| GLR Innovations | +91 9891111994 | New Delhi, India | 4542 | 58 | Inquiry |
| Ganapa Liife Science | +91 7760536535 | Bangalore, India | 168 | 58 | Inquiry |
| REDDY N REDDY PHARMACEUTICALS | +91-9848096759 +91-9848096759 | Hyderabad, India | 1989 | 58 | Inquiry |
| Oceanic Pharmachem Pvt. Ltd. | 91-22-42128600 | Maharashtra, India | 2006 | 58 | Inquiry |
| Vihasibio Sciences Private Limited | -40110230 | Hyderabad, India | 391 | 58 | Inquiry |
| Vyas Bio Life Sciences Pvt. Ltd | -65546373 | Hyderabad, India | 1213 | 58 | Inquiry |
| V & V Pharma Industries | 08068441146Ext 687 | Maharashtra, India | 98 | 58 | Inquiry |
| VASIN BIO LIFE SCIENCES PVT LTD | 9491644688 | Hyderabad, India | 2096 | 58 | Inquiry |
| Dr Chindepalli | 91-7036227677 | Hyderabad, India | 486 | 58 | Inquiry |
| Reax Chemicals | 08048372701Ext 416 | Hyderabad, India | 4223 | 58 | Inquiry |
7254-19-5(5-Bromoindole-2-carboxylic acid)Related Search:
5-BROMOINDOLE-2-CARBOXYLIC ACID METHYL ESTER
5-BROMO-1H-INDOLE-3-CARBOXYLIC ACID METHYL ESTER
4-(4-Bromo-phenyl)-pyridine
5-Bromoindole trimer
5-Bromoindole-2-carboxylic acid
Ethyl 5-Bromoindole-2-carboxylate
6-BROMOINDOLE-2-CARBOXYLIC ACID
5-BROMOINDOLE-3-CARBOXYLIC ACID
4-BROMOINDOLE-1-CARBOXYLIC ACID TERT-BUTYL ESTER
3-BROMOINDOLE-1-CARBOXYLIC ACID TERT-BUTYL ESTER
chevron_left
1of4
chevron_right
5-BROMO-2-INDOLECARBOXYLIC ACID
5-BROMOINDOLE-2-CARBOXYLIC ACID
AKOS JY2082545
1H-INDOLE-2-CARBOXYLIC ACID, 5-BROMO-
5-bromoindole-2-carboxylicacid5-Bromoindole-2-carboxylicacid
5-Bromoindole-2-carboxylic acid ,98%
5-BroMo-1H-indol-2-carboxylic acid
NSC 73384
5-Bromoindazole-2-carboxylic acid
5-Bromo-2-carboxy-1H-indole
5-Bromoindole-2-carboxylicAcid>
5-BROMO-1H-INDOLE-2-CARBOXYLIC ACID
7254-19-5
Br1C9H6N1O2
C9H6BrNO2
Building Blocks
Heterocyclic Building Blocks
Halogenated Heterocycles
Indoles
Heterocycles series
Indole/indoline/oxindole
Indole and Indoline
Indoles and derivatives
pharmacetical
Organic acids
Indoles
Indole
Halogenated Heterocycles
Heterocyclic Building Blocks
IndolesBuilding Blocks
Pharmaceutical Intermediates
Building Blocks
C7 to C10
C7 to C9
Chemical Synthesis
Halogenated Heterocycles
Heterocyclic Building Blocks
1





