Magnesium hydroxide
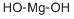
- CAS No.
- 1309-42-8
- Chemical Name:
- Magnesium hydroxide
- Synonyms
- Mg(OH)2;MAGNESIUM HYDRATE;Magnesium hydroxide, Ph.Eur.4, BP2001, USP24, E528;K537;magox;s/g84;FR-20;MGH-93;milmag;Simeco
- CBNumber:
- CB0239262
- Molecular Formula:
- H2MgO2
- Molecular Weight:
- 58.32
- MOL File:
- 1309-42-8.mol
- MSDS File:
- SDS
- Modify Date:
- 2025/1/27 9:38:02
| Melting point | 350 °C (lit.) |
|---|---|
| Density | 2,36 g/cm3 |
| storage temp. | Room Temperature |
| solubility | 5 M HCl: 0.1 M, clear, yellow |
| form | powder |
| Specific Gravity | 2.36 |
| color | White |
| Odor | Odorless |
| PH Range | 9.5 - 10.5 |
| PH | 10.4(1 mM solution);10.4(10 mM solution);10.4(100 mM solution) |
| Water Solubility | 0.9 mg/100 mL (18 ºC) |
| Sensitive | Air Sensitive |
| λmax |
λ: 260 nm Amax: 0.030 λ: 280 nm Amax: 0.025 |
| Merck | 14,5670 |
| Solubility Product Constant (Ksp) | pKsp: 11.25 |
| Stability | Stable. |
| InChIKey | VTHJTEIRLNZDEV-UHFFFAOYSA-L |
| LogP | -1.380 (est) |
| CAS DataBase Reference | 1309-42-8(CAS DataBase Reference) |
| NIST Chemistry Reference | Magnesium hydroxide(1309-42-8) |
| EPA Substance Registry System | Magnesium hydroxide (Mg(OH)2) (1309-42-8) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H315-H319-H335 | |||||||||
| Precautionary statements | P261-P280a-P304+P340-P305+P351+P338-P405-P501a | |||||||||
| Hazard Codes | Xi | |||||||||
| Risk Statements | 36/37/38 | |||||||||
| Safety Statements | 26-36-37/39-24/25 | |||||||||
| WGK Germany | - | |||||||||
| RTECS | OM3570000 | |||||||||
| F | 3-9 | |||||||||
| TSCA | Yes | |||||||||
| HS Code | 28161000 | |||||||||
| Hazardous Substances Data | 1309-42-8(Hazardous Substances Data) | |||||||||
| Toxicity | LD50 oral in rat: 8500mg/kg | |||||||||
| NFPA 704 |
|
Magnesium hydroxide price More Price(20)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | M5421 | Magnesium hydroxide BioXtra, ≥95% | 1309-42-8 | 1KG | ₹9839.93 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 632309 | Magnesium hydroxide nanopowder, <100?nm particle size (laser PSA), 99.8% trace metals basis | 1309-42-8 | 25G | ₹14375.6 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 632309 | Magnesium hydroxide nanopowder, <100?nm particle size (laser PSA), 99.8% trace metals basis | 1309-42-8 | 100G | ₹35765.8 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 63081 | Magnesium hydroxide BioUltra, ≥99.0% (KT) | 1309-42-8 | 250G | ₹6072.83 | 2022-06-14 | Buy |
| Sigma-Aldrich | 63081 | Magnesium hydroxide BioUltra, ≥99.0% (KT) | 1309-42-8 | 1KG | ₹19560.78 | 2022-06-14 | Buy |
Magnesium hydroxide Chemical Properties,Uses,Production
Description
A white, bulky powder. It dissolves in dilute acids, but is practically insoluble in water and in alcohol.
Chemical Properties
Magnesium hydroxide, Mg(OHn, also known as magnesium hydrate and brucite, is a white powder that is very slightly soluble in water. It decomposes at 350°C (662 OF). It is used in the extraction of magnesium metal and as a reagent in the sulfite wood pulp process. Magnesium hydroxide is formed by the reaction of sodium hydroxide and a soluble magnesium salt solution. Brucite, a mineral composed of magnesium hydroxide with occasional traces of iron and manganese, is a white to grayish translucent secondary mineral found with serpentine and metamorphic dolomites.
Physical properties
Magnesium Hydroxide decomposes when heated to 360°C and forms
the oxide, MgO. It is very slightly soluble in water at
0.00122 g/100 ml and has a solubility product of
5.61×10-12. Crystals of Mg(OH)2 have a refractive
index of 1.559. Its heat of formation is –925 kJ/mol
with an entropy of 63 J/mol·K.
As a suspension in water, it is often called milk of
magnesia because of its milk-like appearance. The solid
mineral form of magnesium hydroxide is known as
“brucite”.
It can be prepared by the metathesis reaction
between any soluble magnesium salts and an alkali
hydroxide such as sodium or even ammonium:
Mg2+(aq)+2OH-(aq)→Mg(OH)2(solid)
Magnesium hydroxide is a common component of
antacids and laxatives. It interferes with the absorption
of folic acid and iron. Its low solubility makes it a weak base.
Another method of commercial manufacture of
magnesium hydroxide involves the chloride obtained from seawater. After the chloride is recrystallized
several times to obtain a level of purity, it is calcined at
about 1000°C:
MgCl2·6H2O → MgCl2+6H2O
→ Mg(OH)-xCl2-x+xHCl
→ MgO+(2-x)HCl
Occurrence
Magnesium Hydroxide occurs naturally as the mineral brucite. Brucite, usually found as a low temperature, hydrothermal vein mineral associated with calcite, aragonite, talc, or magnesite, appears as a decomposition product of magnesium silicates associated with serpentine, dolomite, magnesite, and chromite. Brucite also occurs as a hydrated form of periclase, and is found in serpentine, marble, chlorite schists, and in crystalline limestone.
Uses
Magnesium Hydroxide is an alkali that is a general purpose food additive. it exists as a white powder and has poor solubility in water and in alcohol. in frozen desserts it will increase the tendency for fat globules to clump, which results in an increase in dryness. it reacts with triglycerides in fatty acids to form soaps. it also functions as a drying agent in foods.
Definition
ChEBI: A magnesium hydroxide in which the magnesium atom is bound to two hydroxide groups.
Pharmaceutical Applications
The synthesis of magnesium hydroxide [Mg(OH)2]
is based on a metathesis reaction in which magnesium salts are reacted with sodium or potassium hydroxide.
Magnesium hydroxide (Mg(OH)2) has only limited solubility
in water and the resulting suspension is called milk of magnesia, which is commonly used as an antacid and is
known to be a mild base.
Magnesium hydroxide [Mg(OH)2] is present in antacids because of its laxative properties and is also the
main ingredient of the ‘milk of magnesia’. The ‘milk of magnesia’ is a suspension of Mg(OH)2 in water,
which has a milk-like appearance because of the low aqueous solubility of Mg(OH)2. It is considered as a
strong electrolyte and a weak base and is given to the patient for indigestion and heartburn. The alkaline
suspension neutralises any excess stomach acid and therefore works as an antacid. It also stimulates intestinal
movement, as the magnesium ions increases the water content in the intestines through its osmotic effect and
as a result softens any faeces present.
Safety Profile
Moderately toxic by intraperitoneal route. Human systemic effects: chlorine level changes, coma, somnolence. Incompatible with maleic anhydride, phosphorus. See also MAGNESIUM COMPOUNDS.
Magnesium hydroxide Preparation Products And Raw materials
Raw materials
1of2
chevron_rightPreparation Products
1of4
chevron_right| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| ANJI BIOSCIENCES | +91-9000100077 +91-9000100077 | Hyderabad, India | 429 | 58 | Inquiry |
| Yogi Dye Chem Industries | +91-9821098577 +91-9821098577 | Mumbai, India | 124 | 58 | Inquiry |
| S.V ENTERPRISES | +919322701159 | Mumbai, India | 150 | 58 | Inquiry |
| SAANVI CORP | +91-7208939370 +91-7208939370 | Maharashtra, India | 46 | 58 | Inquiry |
| Kronox Lab Sciences Pvt Ltd | +91-9313231074 +91-9313231074 | Gujarat, India | 240 | 58 | Inquiry |
| Sankalp Organics Pvt. Ltd | +919322341858 | Maharashtra, India | 10 | 58 | Inquiry |
| Anish Chemicals | +919429005558 | Gujarat, India | 13 | 58 | Inquiry |
| Sujata Chemicals | +91-9898025496 +91-9898025496 | Gujarat, India | 73 | 58 | Inquiry |
| Taurus Chemicals | +91-8458279503 +91-9849650702 | Telangana, India | 11 | 58 | Inquiry |
| NEELKANTH FINECHEM | +91-9829021177 +91-9928841166 | Rajasthan, India | 34 | 58 | Inquiry |
| Supplier | Advantage |
|---|---|
| ANJI BIOSCIENCES | 58 |
| Yogi Dye Chem Industries | 58 |
| S.V ENTERPRISES | 58 |
| SAANVI CORP | 58 |
| Kronox Lab Sciences Pvt Ltd | 58 |
| Sankalp Organics Pvt. Ltd | 58 |
| Anish Chemicals | 58 |
| Sujata Chemicals | 58 |
| Taurus Chemicals | 58 |
| NEELKANTH FINECHEM | 58 |
Related articles
- The uses and side effects of magnesium hydroxide
- Magnesium hydroxide is a layer-structured mineral, and there are a family of double hydroxides where the magnesium hydroxide l....
- Jun 11,2024
- Magnesium hydroxide: Preparation, applications and pharmacodynamics
- Magnesium hydroxide is the inorganic compound occurring in nature and used in suspension as either an antacid or a laxative, ....
- May 29,2023
- Applications of Magnesium hydroxide
- Magnesium hydroxide is a white to off-white crystalline powder with a specific gravity of 2.4 and a Mohs hardness of around 3.....
- Dec 20,2021
1309-42-8(Magnesium hydroxide)Related Search:
1of4
chevron_right




