Potassium carbonate
- CAS No.
- 584-08-7
- Chemical Name:
- Potassium carbonate
- Synonyms
- K2CO3;Anhydrous potassium carbonate;POTASH;potassium carbonate anhydrous;POTASSIUM CARBONATE USP;Pearl dust;Kaliumcarbonat;BUFFERED PEPTONE;dipotassiumcarbonate;PotassiumCarbonateFcc
- CBNumber:
- CB4853879
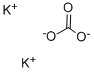
- Molecular Formula:
- K2CO3
- Molecular Weight:
- 138.21
- MOL File:
- 584-08-7.mol
- MSDS File:
- SDS
- Modify Date:
- 2024/6/7 16:47:56
| Melting point | 891 °C (lit.) |
|---|---|
| Boiling point | decomposes [STR93] |
| Density | 2.43 g/mL at 25 °C |
| storage temp. | Store at +5°C to +30°C. |
| solubility | H2O: 1 M at 20 °C, clear, colorless |
| pka | 10.33[at 20 ℃] |
| form | powder |
| Specific Gravity | 2.29 |
| color | Yellow |
| PH | 10.52(1 mM solution);11(10 mM solution);11.36(100 mM solution); |
| Odor | at 100.00?%. odorless |
| Water Solubility | 1120 g/L (20 ºC) |
| Sensitive | Hygroscopic |
| λmax |
λ: 260 nm Amax: 0.03 λ: 280 nm Amax: 0.02 |
| Merck | 14,7619 |
| BRN | 4267587 |
| Dielectric constant | 5.6(16.0℃) |
| Stability | Stable. Incompatible with moisture, acids, magnesium bromine trifluoride and magnesium bromine trichloride. |
| CAS DataBase Reference | 584-08-7(CAS DataBase Reference) |
| NIST Chemistry Reference | Dipotassium carbonate(584-08-7) |
| EPA Substance Registry System | Potassium carbonate (584-08-7) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H315-H319-H335 | |||||||||
| Precautionary statements | P261-P264-P271-P280-P302+P352-P305+P351+P338 | |||||||||
| Hazard Codes | Xn | |||||||||
| Risk Statements | 22-36/37/38-20/21/22 | |||||||||
| Safety Statements | 26-36-37/39 | |||||||||
| RIDADR | 3262 | |||||||||
| WGK Germany | 1 | |||||||||
| RTECS | TS7750000 | |||||||||
| F | 3 | |||||||||
| TSCA | Yes | |||||||||
| PackingGroup | III | |||||||||
| HS Code | 28364000 | |||||||||
| Toxicity | LD50 orally in rats: 1.87 g/kg, H. F. Smyth et al., Am. Ind. Hyg. Assoc. J. 30, 470 (1969) | |||||||||
| NFPA 704 |
|
Potassium carbonate price More Price(68)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | P5833 | Potassium carbonate BioXtra, ≥99.0% | 584-08-7 | 1KG | ₹7620.8 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 900502 | Potassium carbonate anhydrous, free-flowing, ?325?mesh, Redi-Dri?, reagent grade, ≥98% | 584-08-7 | 500G | ₹7166.15 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | P5833 | Potassium carbonate BioXtra, ≥99.0% | 584-08-7 | 500G | ₹8486.8 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 900502 | Potassium carbonate anhydrous, free-flowing, ?325?mesh, Redi-Dri?, reagent grade, ≥98% | 584-08-7 | 1KG | ₹12286.38 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 900502 | Potassium carbonate anhydrous, free-flowing, ?325?mesh, Redi-Dri?, reagent grade, ≥98% | 584-08-7 | 2.5KG | ₹20924.73 | 2022-06-14 | Buy |
Potassium carbonate Chemical Properties,Uses,Production
Chemical Properties
Potassium carbonate is a white, crystalline, salt that forms basic aqueous solutions used in the production of fertilizer, glass, ceramics, explosives, soaps, chemicals, and wool treatments. It was the main compound once referred to as potash, although the term today is not reserved exclusively for potassium carbonate, but for several potassium salts. In the fertilizer industry, potash refers to potassium oxide, K2O, rather than potassium carbonate. Pearlash is a purer form of potash made by heating potash to remove impurities.
Uses
Potassium carbonate is used in the chemical industry as a source of inorganic potassium salts (potassium silicates, potassium bicarbonate), which are used in fertilizers, soaps, adhesives, dehydrating agents, dyes, and pharmaceuticals. Potassium carbonate used to make potassium lye produces soft soaps, which are liquids or semisolids rather than solids. Other uses of potassium carbonate includes use as a fire suppressant in extinguishers, as a CO2 absorbent for chemical processes and pollution control, an antioxidant in rubber additives, and in pharmaceutical formulations.
Definition
ChEBI: Potassium carbonate is a potassium salt that is the dipotassium salt of carbonic acid. It has a role as a catalyst, a fertilizer and a flame retardant. It is a carbonate salt and a potassium salt.
Production Methods
Potassium carbonate can be obtained from ash or by electrolysis of potassium chloride. Another method involves reacting potassium hydroxide with carbon dioxide. The resulting liquid carbonate contains approximately 50% potassium carbonate in water. However, the solid product, which contains over 70% potassium carbonate, is expensive and only suitable for certain types of acidic soil. Additionally, neutralizing caustic potash with carbon dioxide also yields potassium carbonate.
General Description
Potassium carbonate (K2CO3) is a white salt, soluble in water (insoluble in ethanol) which forms a strongly alkaline solution. It can be made as the product of potassium hydroxide's absorbent reaction with carbon dioxide. It presents a large capacity to absorb moisture.Corrosive to metals and tissue. Density 12.8 lb /gal. Used to make soaps, other potassium compounds, in liquid fertilizers.
Air & Water Reactions
Water soluble. Addition of water evolves heat.
Reactivity Profile
Potassium carbonate neutralizes acids exothermically to form salts plus water. Reacts with certain metals (such as aluminum and zinc) to form oxides or hydroxides of the metal and generate gaseous hydrogen. May initiate polymerization reactions in polymerizable organic compounds, especially epoxides. May generate flammable and/or toxic gases with ammonium salts, nitrides, halogenated organics, various metals, peroxides, and hydroperoxides. May serve as a catalyst. Reacts when heated above about 84°C with aqueous solutions of reducing sugars other than sucrose, to evolve toxic levels of carbon monoxide [Bretherick, 5th Ed., 1995].
Hazard
Solutions irritating to tissue.
Health Hazard
TOXIC; inhalation, ingestion or skin contact with material may cause severe injury or death. Contact with molten substance may cause severe burns to skin and eyes. Avoid any skin contact. Effects of contact or inhalation may be delayed. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.
Fire Hazard
Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Some are oxidizers and may ignite combustibles (wood, paper, oil, clothing, etc.). Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated.
Safety Profile
Poison by ingestion. A strong caustic. Incompatible with KCO, chlorine trifluoride, magnesium. Mutation data reported. When heated to decomposition it emits toxic fumes of K2O.
Purification Methods
It crystallises from water between 100o and 0o. The solubility in H2O is 105% at 0o, 127% at 60o and 205% at 135o (b of saturated solution). After two recrystallisations of technical grade material, it had B, Li and Fe at 1.0, 0.04 and 0.01 ppm, respectvely. [D.nges in Handbook of Preparative Inorganic Chemistry (Ed. Brauer) Academic Press Vol I p 987 1963.]
References
https://en.wikipedia.org/wiki/Potassium_carbonate
https://www.drugs.com/inactive/potassium-carbonate-106.html
http://www.sciencelab.com/msds.php?msdsId=9926681
https://pubchem.ncbi.nlm.nih.gov/compound/Potassium-Carbonate
Solubility of Potassium Carbonate and Potassium Hydrocarbonate in Methanol DOI:10.1021/JE020012V
Potassium carbonate Preparation Products And Raw materials
Raw materials
1of2
chevron_rightPreparation Products
1of8
chevron_right| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| CLEARSYNTH LABS LTD. | +91-22-45045900 | Mumbai, India | 66 | 58 | Inquiry |
| ANJI BIOSCIENCES | +91-9000100077 +91-9000100077 | Hyderabad, India | 430 | 58 | Inquiry |
| Sudarshan Pharma Industries Limited | +91-2242221111 +91-9320932107 | Maharashtra, India | 37 | 58 | Inquiry |
| Vardhaman P Golechha | 9704422000 | Telangana, India | 294 | 58 | Inquiry |
| PAARICHEM RESOURCES LLP | +91-8104961021 +91-8104961021 | Maharashtra, India | 82 | 58 | Inquiry |
| JSK Chemicals | +919879767970 | Gujarat, India | 3756 | 58 | Inquiry |
| SAANVI CORP | +91-7208939370 +91-7208939370 | Maharashtra, India | 46 | 58 | Inquiry |
| Kronox Lab Sciences Pvt Ltd | +91-9313231074 +91-9313231074 | Gujarat, India | 240 | 58 | Inquiry |
| Sree Rayalaseema HI Strength Hypo Ltd | +91-9848867692 +91-9848867692 | Andhra Pradesh, India | 19 | 58 | Inquiry |
| Merck Ltd | +91-2262109800 +91-2262109000 | Maharashtra, India | 272 | 58 | Inquiry |
| Supplier | Advantage |
|---|---|
| CLEARSYNTH LABS LTD. | 58 |
| ANJI BIOSCIENCES | 58 |
| Sudarshan Pharma Industries Limited | 58 |
| Vardhaman P Golechha | 58 |
| PAARICHEM RESOURCES LLP | 58 |
| JSK Chemicals | 58 |
| SAANVI CORP | 58 |
| Kronox Lab Sciences Pvt Ltd | 58 |
| Sree Rayalaseema HI Strength Hypo Ltd | 58 |
| Merck Ltd | 58 |
Related articles
- Potassium carbonate: a food additive
- One of the uses of potassium carbonate is an additive in producing some foods. Additives are essential compounds for the produ....
- Apr 15,2024
- Potassium carbonate: Preparation, application, quantitative method and toxicity
- Potassium carbonate is the primary component of potash and the more refined pearl ash or salts of tartar. It is prepared comme....
- Apr 3,2023
- Handling of Liquid Potassium Carbonate
- Liquid 47% potassium carbonate is typically shipped in drums, tank truck or rail car. It is also available by consignment as a....
- Nov 15,2021





