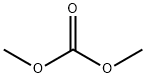
Dimethyl carbonate
- CAS No.
- 616-38-6
- Chemical Name:
- Dimethyl carbonate
- Synonyms
- DIMETHYL CARBONATE;METHYL CARBONATE;CARBONIC ACID DIMETHYL ESTER;Dimethylcarbonat;dimetyl carbonate;methylcarbonate((meo)2co);CH3OCOOCH3;Dimethylcarbonate,99%;DMC;DMC
- CBNumber:
- CB8853983
- Molecular Formula:
- C3H6O3
- Molecular Weight:
- 90.08
- MOL File:
- 616-38-6.mol
- MSDS File:
- SDS
- Modify Date:
- 2024/7/2 14:09:54
| Melting point | 2-4 °C (lit.) |
|---|---|
| Boiling point | 90 °C (lit.) |
| Density | 1.069 g/mL at 25 °C (lit.) |
| vapor density | 3.1 (vs air) |
| vapor pressure | 18 mm Hg ( 21.1 °C) |
| refractive index |
n |
| Flash point | 65 °F |
| storage temp. | Store below +30°C. |
| solubility | 139g/l |
| form | Liquid |
| color | <50(APHA) |
| Odor | Pleasant |
| explosive limit | 4.22-12.87%(V) |
| Water Solubility | 139 g/L |
| Sensitive | Moisture Sensitive |
| Merck | 14,3241 |
| BRN | 635821 |
| Dielectric constant | 3.1699999999999999 |
| Stability | Stable. Highly flammable. Incompatible with strong oxidizing agents, potassium t-butoxide. |
| InChIKey | IEJIGPNLZYLLBP-UHFFFAOYSA-N |
| LogP | 0.23-0.354 at 20-25℃ |
| CAS DataBase Reference | 616-38-6(CAS DataBase Reference) |
| NIST Chemistry Reference | Carbonic acid, dimethyl ester(616-38-6) |
| EPA Substance Registry System | Carbonic acid, dimethyl ester (616-38-6) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS02 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H225 | |||||||||
| Precautionary statements | P210-P233-P240-P241-P242-P243 | |||||||||
| Hazard Codes | F | |||||||||
| Risk Statements | 11 | |||||||||
| Safety Statements | 9-16-2017/9/16 | |||||||||
| RIDADR | UN 1161 3/PG 2 | |||||||||
| WGK Germany | 1 | |||||||||
| RTECS | FG0450000 | |||||||||
| Autoignition Temperature | 458 °C | |||||||||
| TSCA | Yes | |||||||||
| HS Code | 2920 90 10 | |||||||||
| HazardClass | 3 | |||||||||
| PackingGroup | II | |||||||||
| Toxicity | LD50 in rats (g/kg): 13.8 orally; 2.5 dermally; LC50 (4 hr) in rats (mg/l): 140 by inhalation (Ono) | |||||||||
| NFPA 704 |
|
Dimethyl carbonate price More Price(21)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | D152927 | Dimethyl carbonate ReagentPlus?, 99% | 616-38-6 | 500ML | ₹4178.45 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | D152927 | Dimethyl carbonate ReagentPlus?, 99% | 616-38-6 | 1L | ₹7566.68 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 809942 | Dimethyl carbonate ≥99.9%, acid <10 ppm, H2O <10 ppm | 616-38-6 | 25G | ₹20134.5 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 809942 | Dimethyl carbonate ≥99.9%, acid <10 ppm, H2O <10 ppm | 616-38-6 | 500G | ₹70254.25 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 8.03525 | Dimethyl carbonate for synthesis | 616-38-6 | 100ML | ₹4290 | 2022-06-14 | Buy |
Dimethyl carbonate Chemical Properties,Uses,Production
Uses
Environmentally benign substitute for dimethyl sulfate, q.v., and methyl halides in methylation reactions and for phosgene, q.v., in methylcarbonylation reactions.
Definition
ChEBI: Dimethyl carbonate is a carbonate ester that is carbonic acid in which both hydrogens are replaced by methyl groups. A flammable, colourless liquid (m.p. 2-4°C, b.p. 90°C) with a characterstic ester-like odour, it is used as a 'green' methylating agent and as a solvent. It has a role as a solvent and a reagent.
Preparation
Dimethyl carbonate (DMC) is formed by the reaction of MeOH with phosgene or methyl chloroformate in the presence of a concentrated sodium hydroxide solution in a two-phase reaction in high yields and purity. Other alcohols can also be phosgenated. As DMC is now more easily accessible via the direct oxidative carbonylation of MeOH, phosgenation is losing its attractiveness in this application.
General Description
Dimethyl carbonate appears as a clear, colorless liquid with a pleasant odor. Denser than water and slightly soluble in water. Vapors are heavier than air. Used to make other chemicals and as a special purpose solvent.
Air & Water Reactions
Highly flammable. Slightly soluble in water.
Reactivity Profile
Dimethyl carbonate reacts with acids to liberate heat along with methanol and carbon dioxide. Strong oxidizing acids may cause a vigorous reaction that is sufficiently exothermic to ignite the reaction products. Heat is also generated by the interaction with caustic solutions. Flammable hydrogen is generated by mixing with alkali metals and hydrides.
Hazard
Flammable, dangerous fire risk. Toxic by inhalation, strong irritant.
Health Hazard
May cause toxic effects if inhaled or absorbed through skin. Inhalation or contact with material may irritate or burn skin and eyes. Fire will produce irritating, corrosive and/or toxic gases. Vapors may cause dizziness or suffocation. Runoff from fire control or dilution water may cause pollution.
Fire Hazard
HIGHLY FLAMMABLE: Will be easily ignited by heat, sparks or flames. Vapors may form explosive mixtures with air. Vapors may travel to source of ignition and flash back. Most vapors are heavier than air. They will spread along ground and collect in low or confined areas (sewers, basements, tanks). Vapor explosion hazard indoors, outdoors or in sewers. Runoff to sewer may create fire or explosion hazard. Containers may explode when heated. Many liquids are lighter than water.
Safety Profile
Moderately toxic by intraperitoneal route. Mildly toxic by ingestion. An irritant. Violent reaction or ignition on contact with potassium-tertbutoxide. A very dangerous fire hazard when exposed to heat, open flames (sparks), or oxiduers. To fight fire, use alcohol foam. When heated to decomposition it emits acrid smoke and irritating fumes.
Purification Methods
If the reagent has broad intense bands at 3300cm-1 and above (i.e. OH stretching), then it should be purified further. Wash it successively with 10% Na2CO3 solution, saturated CaCl2, H2O, and dry it by shaking mechanically for 1hour with anhydrous CaCl2, and fractionate. [Bowden & Butler J Chem Soc 78 1939, Vogel J Chem Soc 1847 1948, Beilstein 3 IV 3.]
Dimethyl carbonate Preparation Products And Raw materials
Raw materials
1of2
chevron_rightPreparation Products
1of5
chevron_right| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| GLR Innovations | +91 9891111994 | New Delhi, India | 4542 | 58 | Inquiry |
| Soham Chemical Industries | +91-7016081644 +91-7016081644 | Mumbai, India | 83 | 58 | Inquiry |
| PAARICHEM RESOURCES LLP | +91-8104961021 +91-8104961021 | Maharashtra, India | 82 | 58 | Inquiry |
| JSK Chemicals | +919879767970 | Gujarat, India | 3756 | 58 | Inquiry |
| oswal chemicals | +91-9173644055 +91-9173644055 | Gujarat, India | 66 | 58 | Inquiry |
| Chem stride | +91-8169461298 +91-8169461298 | Maharashtra, India | 27 | 58 | Inquiry |
| Otto Chemie Pvt Ltd | +91-2222070099 +91-2266382599 | Maharashtra, India | 429 | 58 | Inquiry |
| Merck Ltd | +91-2262109800 +91-2262109000 | Maharashtra, India | 272 | 58 | Inquiry |
| Jiya Pharmachem | 08068442303Ext 473 | Mumbai, India | 83 | 58 | Inquiry |
| Shanti Chemical Works | 08048970703 | Karnataka, India | 5 | 58 | Inquiry |
| Supplier | Advantage |
|---|---|
| GLR Innovations | 58 |
| Soham Chemical Industries | 58 |
| PAARICHEM RESOURCES LLP | 58 |
| JSK Chemicals | 58 |
| oswal chemicals | 58 |
| Chem stride | 58 |
| Otto Chemie Pvt Ltd | 58 |
| Merck Ltd | 58 |
| Jiya Pharmachem | 58 |
| Shanti Chemical Works | 58 |
Related articles
- The wide range of applications: Dimethyl carbonate
- Dimethyl carbonate is a versatile compound that represents an attractive, eco-friendly alternative to both methyl halides (or ....
- Apr 25,2024
- Dimethyl Carbonate: Production and Hazards
- The passage introduces the production and hazards of Dimethyl carbonate.
- Nov 28,2022
616-38-6(Dimethyl carbonate)Related Search:
1of4
chevron_right




