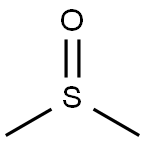
Dimethyl sulfoxide
- CAS No.
- 67-68-5
- Chemical Name:
- Dimethyl sulfoxide
- Synonyms
- DMSO;DIMETHYL SULPHOXIDE;METHYL SULFOXIDE;suL;Dimexide;(CH3)2SO;Sclerosol;METHYL SULPHOXIDE;dimethylsulfoxyde;dimethyl sulfoxide, anhydrous
- CBNumber:
- CB7854105
- Molecular Formula:
- C2H6OS
- Molecular Weight:
- 78.13
- MOL File:
- 67-68-5.mol
- MSDS File:
- SDS
- Modify Date:
- 2024/6/4 19:18:49
| Melting point | 18.4 °C |
|---|---|
| Boiling point | 189 °C(lit.) |
| Density | 1.100 g/mL at 20 °C |
| vapor density | 2.7 (vs air) |
| vapor pressure | 0.42 mm Hg ( 20 °C) |
| FEMA | 3875 | METHYLSULFINYLMETHANE |
| refractive index |
n |
| Flash point | 192 °F |
| storage temp. | Store at +5°C to +30°C. |
| solubility | H2O: miscible (completely) |
| pka | 35(at 25℃) |
| form | liquid (temperature dependent) |
| color | clear colorless |
| Relative polarity | 0.444 |
| Odor | Mild garlic odor |
| explosive limit | 1.8-63.0%(V) |
| Odor Type | alliaceous |
| Water Solubility | Soluble in water, methanol, acetone, ether, benzene, chloroform. |
| FreezingPoint | 18.4℃ |
| Sensitive | Hygroscopic |
| λmax |
λ: 285 nm Amax: ≤0.20 λ: 295 nm Amax: ≤0.20 |
| JECFA Number | 507 |
| Merck | 14,3259 |
| BRN | 506008 |
| Dielectric constant | 41.9(55℃) |
| Stability | Stable. Incompatible with a very wide range of materials, including acid chlorides, strong acids, strong oxidizing agents, strong reducing agents, phosphorus halides, moisture, copper wool + trichloroacetic acid. Reacts violently with a number of materials - consult a full data sheet before use. Hygroscopic. |
| InChIKey | IAZDPXIOMUYVGZ-UHFFFAOYSA-N |
| LogP | -1.35 |
| CAS DataBase Reference | 67-68-5(CAS DataBase Reference) |
| NIST Chemistry Reference | Dimethyl sulfoxide(67-68-5) |
| EPA Substance Registry System | Dimethyl sulfoxide (67-68-5) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS08 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H227-H320-H373 | |||||||||
| Precautionary statements | P210e-P280a-P370+P378a-P403+P235-P501a-P260-P264-P305+P351+P338+P337+P313-P314-P501 | |||||||||
| Hazard Codes | Xi | |||||||||
| Risk Statements | 36/37/38 | |||||||||
| Safety Statements | 24/25-37/39-26-36-23 | |||||||||
| RIDADR | NA 1993 / PGIII | |||||||||
| WGK Germany | 1 | |||||||||
| RTECS | PV6210000 | |||||||||
| F | 3 | |||||||||
| Autoignition Temperature | 215 °C | |||||||||
| TSCA | Yes | |||||||||
| HS Code | 29309070 | |||||||||
| Toxicity | LD50 orally in rats: 17.9 ml/kg (Bartsch) | |||||||||
| NFPA 704 |
|
Dimethyl sulfoxide price More Price(170)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | W387520 | Methyl sulfoxide ≥99%, FG | 67-68-5 | 1SAMPLE | ₹5141.88 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | W387520 | Methyl sulfoxide ≥99%, FG | 67-68-5 | 1KG | ₹7361 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | W387520 | Methyl sulfoxide ≥99%, FG | 67-68-5 | 10KG | ₹20318.53 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | W387520 | Methyl sulfoxide ≥99%, FG | 67-68-5 | 25KG | ₹43105.15 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | D8418 | Dimethyl sulfoxide for molecular biology | 67-68-5 | 50ML | ₹6808.93 | 2022-06-14 | Buy |
Dimethyl sulfoxide Chemical Properties,Uses,Production
Description
First synthesized in 1866 by Alexander Zaytsev in the Russian Empire, dimethyl sulfoxide is an organosulfur compound. This colorless liquid is an important polar aprotic solvent that dissolves both polar and nonpolar compounds and is miscible in a wide range of organic solvents as well as water.
Chemical Properties
Dimethyl sulfoxide occurs as a colorless, viscous liquid, or as colorless crystals that are miscible with water, alcohol, and ether. The material hasa slightly bitter taste with a sweet aftertaste, and is odorless, or has a slight odor characteristic of dimethyl sulfoxide.Dimethyl sulfoxide is extremely hygroscopic, absorbing up to 70% of its own weight in water with evolution of heat.
Occurrence
Reported found in apple, raspberry, cabbage, cucumber, onion, tomato, peppermint and spearmint oils, milk, pork liver, beer, rum, cocoa, coffee, black tea, oatmeal, soybean, beetroot, parsnip root, watercress, sweet corn, malt, cooked shrimp and oysters
Uses
Dimethyl sulfoxide (1-10%) has been shown to accelerate strand renaturation and is believed to give the nucleic acid thermal stability against depurination. As a PCR cosolvent, DMSO may help improve yields, especially in long PCR.
Production Methods
Dimethyl sulfoxide is prepared by air oxidation of dimethyl sulfide in the presence of nitrogen oxides. It can also be obtained as a by product of wood pulp manufacture for the paper and allied industries.
Definition
ChEBI: A 2-carbon sulfoxide in which the sulfur atom has two methyl substituents.
General Description
A clear liquid, essentially odorless. Closed cup flash point 192°F. Vapors are heavier than air. Contact with the skin may cause stinging and burning and lead to an odor of garlic on the breath. An excellent solvent that can transport toxic solutes through the skin. High vapor concentrations may cause headache, dizziness, and sedation.
Air & Water Reactions
Denser than water and miscible in water.
Reactivity Profile
Dimethyl sulfoxide decomposes violently on contact with many acyl halides, aryl halides and related compounds such as phenyl and tolyl chloride, acetyl chloride, benzenesulfonyl chloride, benzoyl chloride, cyanuric chloride, phosphorus chloride, phosphorus oxychloride, and thionyl chloride [Chem. Eng. News 35(9):87 (1957)]. Reacts, possibly violently, with iodine pentafluoride [Chem. Eng. News 47(12):, 109(1969)]. Vacuum distillation from anhydrous magnesium perchlorate led to an explosion [MCA Case History 1187(1966)]. Violently reactive with fluorinating agents such as silver fluoride [Chem. Eng. News 44(24):7(1956)]. Can explode with sodium hydride [Chem. Eng. News 44(24):7(1966)]. Mixture with methyl bromide resulted in an explosion that shattered the apparatus [NFPA 491M, 1991]. Forms salts with perchloric acid that are explosive when dry [Chem. Abst. 44:p3935d (1950)]. Decomposes when heated above normal boiling point.
Health Hazard
The acute toxicity of DMSO by all routes of exposure is very low. Inhalation of DMSO vapor can cause irritation of the respiratory tract, and at higher concentrations may cause vomiting, chills, headache, and dizziness. The material is only slightly toxic by ingestion and may cause vomiting, abdominal pain, and lethargy. Dimethyl sulfoxide is relatively nontoxic by skin absorption, but can cause itching, scaling, and a transient burning sensation. Dimethyl sulfoxide can increase the tendency for other chemicals to penetrate the skin and so increase their toxic effects. Contact of DMSO liquid with the eyes may cause irritation with redness, pain, and blurred vision. Chronic exposure to dimethyl sulfoxide can cause damage to the cornea of the eye. Dimethyl sulfoxide has not been found to be carcinogenic or to show reproductive or developmental toxicity in humans
Flammability and Explosibility
Combustible when exposed to heat or flame (NFPA rating = 1). Carbon dioxide or dry chemical extinguishers should be used to fight DMSO fires.
Chemical Reactivity
Reactivity with Water No reaction; Reactivity with Common Materials: No reaction; Stability During Transport: Stable; Neutralizing Agents for Acids and Caustics: Not pertinent; Polymerization: Not pertinent; Inhibitor of Polymerization: Not pertinent.
Biological Activity
Solvent with wide ranging applications in biological research.
Environmental Fate
Most physiological properties of DMSO appear to be related to its penetration properties, its potential to inhibit or stimulate enzymes and to act as a free radical scavenger, and its ability to cause histamine release from mast cells. These properties are largely based on DMSO’s chemical characteristics, including its hydrogen bonding behavior, water affinity, ability to interchange with water in membranes, and ability to react with organic molecules.
storage
Dimethyl sulfoxide is reasonably stable to heat,but upon prolonged reflux it decomposes slightly to methyl mercaptan and bismethylthiomethane. This decomposition is aided by acids, and is retarded by many bases. When heated to decomposition, toxic fumes are emitted.
At temperatures between 40 and 60°C, it has been reported that dimethyl sulfoxide suffers a partial breakdown, which is indicated by changes in physical properties such as refractive index, density, and viscosity.
Dimethyl sulfoxide should be stored in airtight, light-resistant containers. The PhEur 6.0 states that glass containers should be used. Contact with plastics should be avoided.
Purification Methods
This colourless, odourless, very hygroscopic liquid, is synthesised from dimethyl sulfide. The main impurity is water, with a trace of dimethyl sulfone. The Karl-Fischer test is applicable. It is dried with Linde types 4A or 13X molecular sieves, by prolonged contact and passage through a column of the material, then distilled under reduced pressure. Other drying agents include CaH2, CaO, BaO and CaSO4. It can also be fractionally crystallised by partial freezing. More extensive purification is achieved by standing overnight with freshly heated and cooled chromatographic grade alumina. It is then refluxed for 4hours over CaO, dried over CaH2, and fractionally distilled at low pressure. For efficiency of desiccants in drying dimethyl sulfoxide see Burfield and Smithers [J Org Chem 43 3966 1978, Sato et al. J Chem Soc, Dalton Trans 1949 1986]. [Reddy Pure Appl Chem 25 459 1969, Beilstein 1 IV 1277.] Rapid purification: Stand over freshly activated alumina, BaO or CaSO4 overnight. Filter and distil it over CaH2 under reduced pressure (~ 12 mm Hg). Store it over 4A molecular sieves.
Incompatibilities
DMSO reacts violently with strong oxidizers, many acyl halides, boron hydrides, and alkali metals. DMSO can form explosive mixtures with metal salts of oxoacids (sodium perchlorate, iron(III) nitrate).
Waste Disposal
Excess dimethyl sulfoxide and waste material containing this substance should be placed in an appropriate container, clearly labeled, and handled according to your institution's waste disposal guidelines.
Regulatory Status
Included in the FDA Inactive Ingredients Database(IVinfusions,SC implants, and topical preparations). Available in the USA as a 50% solution for irrigation in the treatment of interstitial cystitis. Also available in Canada as a 70% solution for use as a topical antifibrotic, and in Germany as a topical gel containing 10% dimethyl sulfoxide for the treatment of musculoskeletal and joint disorders. Included in topical formulations of idoxuridine and diclofenac licensed in the UK.
Dimethyl sulfoxide Preparation Products And Raw materials
Raw materials
1of2
chevron_rightPreparation Products
1of8
chevron_right| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| CLEARSYNTH LABS LTD. | +91-22-45045900 | Mumbai, India | 66 | 58 | Inquiry |
| ANJI BIOSCIENCES | +91-9000100077 +91-9000100077 | Hyderabad, India | 430 | 58 | Inquiry |
| Sudarshan Pharma Industries Limited | +91-2242221111 +91-9320932107 | Maharashtra, India | 37 | 58 | Inquiry |
| ADVENT CHEMBIO PRIVATE LIMITED | +91-9821524116 +91-9821524116 | Mumbai, India | 353 | 58 | Inquiry |
| Cefa-Cilinas Biotics Pvt Ltd | +91-7875033155 +91-8080701561 | Maharashtra, India | 121 | 58 | Inquiry |
| JSK Chemicals | +919879767970 | Gujarat, India | 3756 | 58 | Inquiry |
| Medi Pharma Drug House | +919930911911 | Mumbai, India | 143 | 58 | Inquiry |
| Dayaram Pharma Chem | +91-9601766800 +91-9601766800 | Gujarat, India | 61 | 58 | Inquiry |
| Merck Ltd | +91-2262109800 +91-2262109000 | Maharashtra, India | 272 | 58 | Inquiry |
| Runa Chemicals Pvt Ltd | +91-9870496650 +91-8422987924 | Maharashtra, India | 4 | 58 | Inquiry |
| Supplier | Advantage |
|---|---|
| CLEARSYNTH LABS LTD. | 58 |
| ANJI BIOSCIENCES | 58 |
| Sudarshan Pharma Industries Limited | 58 |
| ADVENT CHEMBIO PRIVATE LIMITED | 58 |
| Cefa-Cilinas Biotics Pvt Ltd | 58 |
| JSK Chemicals | 58 |
| Medi Pharma Drug House | 58 |
| Dayaram Pharma Chem | 58 |
| Merck Ltd | 58 |
| Runa Chemicals Pvt Ltd | 58 |
Related articles
- Dimethyl Sulfoxide: A Widely Used Polar Aprotic Solvent
- Dimethyl Sulfoxide (DMSO) is a polar aprotic molecule that has the peculiar property of having a mildly nucleophilic sulfur an....
- Dec 27,2023
- Is Dimethyl sulfoxideharmful to Humans?
- DMSO can cause contaminants, toxins, and medicines to be absorbed through the skin, which may cause unexpected effects.
- Dec 1,2022
- Toxicity of Dimethyl sulfoxide
- First synthesized in 1866 by Alexander Zaytsev in the Russian Empire, dimethyl sulfoxide is an organosulfur compound. This col....
- Jan 19,2022
67-68-5(Dimethyl sulfoxide)Related Search:
1of4
chevron_right




