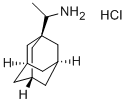
1-AdaMantanethylaMine
- CAS No.
- 13392-28-4
- Chemical Name:
- 1-AdaMantanethylaMine
- Synonyms
- RIMANTADINE;LEVOFLOXACIN HCL;RiMantidine;1-Adamantanemethylamine;RiMantadine (FluMadine);1-Adaman;AKOS B022266;1-Rimantadine;AKOS NCG1-0034;1-Adamantanethylamine
- CBNumber:
- CB7122907
- Molecular Formula:
- C12H21N
- Molecular Weight:
- 179.3
- MOL File:
- 13392-28-4.mol
- MSDS File:
- SDS
- Modify Date:
- 2024/11/19 23:02:33
| Boiling point | 248°C |
|---|---|
| Density | 1.033 |
| Flash point | 99°C |
| storage temp. | Store at -20°C |
| solubility | Soluble in DMSO |
| form | Liquid |
| pka | 11.17±0.29(Predicted) |
| color | Colorless to light yellow |
| CAS DataBase Reference | 13392-28-4(CAS DataBase Reference) |
| NIST Chemistry Reference | 1-Adamantanemethylamine, «alpha»-methyl-(13392-28-4) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|---|---|
| Signal word | Warning |
| Hazard statements | H302-H315-H319-H335 |
| Precautionary statements | P261-P280-P301+P312-P302+P352-P305+P351+P338 |
| HazardClass | IRRITANT |
| HS Code | 2902190000 |
| Hazardous Substances Data | 13392-28-4(Hazardous Substances Data) |
1-AdaMantanethylaMine Chemical Properties,Uses,Production
Uses
Rimantadine act by blocking the M2 ion channel which is required for uptake of protons into the interior of the virus to permit acid-promoted viral uncoating (decapsidation).
General Description
Resistant variants of influenza type A have been recoveredfrom rimantadine-treated patients.Resistance with inhibitory concentrations increased morethan 100-fold have been associated with single nucleotidechanges that lead to amino acid substitutions in the transmembranedomain of M2. rimantadineshare cross-susceptibility and resistance.
Pharmaceutical Applications
An analog of amantadine, supplied as the hydrochloride for oral administration.
Mechanism of action
Rimantadine hydrochloride (α-methyl-1-adamantanemethylamine hydrochloride) is a synthetic adamatane derivative that is structurally and pharmacologically related to amantadine. It appears to be more effective than amantadine hydrochloride against influenza A, with fewer CNS side effects. Rimantadine hydrochloride is thought to interfere with virus uncoating by inhibiting the release of specific proteins. It may act by inhibiting RT or the synthesis of virus-specific RNA, but it does not inhibit virus adsorption or penetration. It appears to produce a virustatic effect early in the virus replication. It is used widely in Russia and Europe.
Pharmacokinetics
Oral absorption: >90%
Cmax 100 mg oral (every 12 h): 0.4–0.5 mg/L after 2–6 h
Plasma half-life: c. 35 h
Volume of distribution: Very large
Plasma protein binding: c. 40%
Absorption and distribution
Single- and multiple-dose pharmacokinetic studies in elderly patients and young adults are remarkably similar. The steadystate concentration in nasal mucus develops by day 5 at a concentration approximately 1.5-fold higher than plasma.
Metabolism
In contrast to amantadine, rimantadine is extensively metabolized in the liver by hydroxylation and glucuronidation.
Excretion
Less than 20% is excreted unchanged in the urine and most of the breakdown products are excreted by this route. Thus, the plasma half-life is much less affected by renal dysfunction than that of amantadine.
Clinical Use
Prophylaxis and treatment of influenza A H1N1 infections Since prolonged administration is well tolerated by elderly patients, the drug is preferable to amantadine.
Side effects
Rimantadine has significantly fewer side effects than amantadine at equivalent doses, perhaps because of differences in pharmacokinetics, since with equal doses the blood levels are considerably lower. CNS side effects are not significantly higher than placebo.
1-AdaMantanethylaMine Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| SynZeal Research Pvt Ltd | +1 226-802-2078 | Gujarat, India | 6514 | 58 | Inquiry |
| Hebei Chuanghai Biotechnology Co., Ltd | +8615531157085 | China | 8808 | 58 | Inquiry |
| Hebei Mujin Biotechnology Co.,Ltd | +86 13288715578 +8613288715578 | China | 12809 | 58 | Inquiry |
| career henan chemical co | +86-0371-86658258 +8613203830695 | China | 29859 | 58 | Inquiry |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | China | 49732 | 58 | Inquiry |
| Guangzhou Yuheng Pharmaceutical Technology Co., Ltd | +8613580539051 | CHINA | 21142 | 58 | Inquiry |
| SIMAGCHEM CORP | +86-13806087780 | China | 17365 | 58 | Inquiry |
| Hubei Ipure Biology Co., Ltd | +8613367258412 | China | 10244 | 58 | Inquiry |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418671 +8618949823763 | China | 34563 | 58 | Inquiry |
| Shanghai UCHEM Inc. | +862156762820 +86-13564624040 | China | 7906 | 58 | Inquiry |
Related articles
- Mechanism and toxicity of Rimantadine
- Rimantadine is an orally administered antiviral drug used to treat, and in rare cases prevent, influenzavirus A infection. Whe....
- Apr 11,2022
13392-28-4(1-AdaMantanethylaMine)Related Search:
1of4
chevron_right




