Silver carbonate
- CAS No.
- 534-16-7
- Chemical Name:
- Silver carbonate
- Synonyms
- SILVER(I) CARBONATE;Silver carbonate on celite;Silver(II) carbonate;Silver Carbonate on Celite (~40 wt. % loading);99.50%;Ag2CO3;CID 13955575;ver carbonate;Silvecarbonate;SILVER CARBONATE
- CBNumber:
- CB5118836
- Molecular Formula:
- CAg2O3
- Molecular Weight:
- 275.75
- MOL File:
- 534-16-7.mol
- MSDS File:
- SDS
- Modify Date:
- 2025/1/27 9:38:02
| Melting point | 210 °C (dec.) (lit.) |
|---|---|
| Density | 6.08 g/mL at 25 °C (lit.) |
| storage temp. | Keep in dark place,Inert atmosphere,Room temperature |
| solubility | Aqueous Acid (Slightly), Aqueous Base (Slightly) |
| form | Granular Powder |
| Specific Gravity | 6.08 |
| color | Green-yellow to greenish |
| Water Solubility | insoluble(3.489×10-3/100ml/20℃) |
| Sensitive | Light Sensitive |
| Merck | 14,8507 |
| Solubility Product Constant (Ksp) | pKsp: 11.07 |
| BRN | 6936654 |
| Stability | Stability Stable, but light sensitive. Incompatible with reducing agents, acids. |
| InChIKey | KQTXIZHBFFWWFW-UHFFFAOYSA-L |
| CAS DataBase Reference | 534-16-7(CAS DataBase Reference) |
| NIST Chemistry Reference | silver carbonate(534-16-7) |
| EPA Substance Registry System | Silver(I) carbonate (534-16-7) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS05,GHS09 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H318-H410 | |||||||||
| Precautionary statements | P273-P280-P305+P351+P338-P391-P501 | |||||||||
| Hazard Codes | Xn,Xi | |||||||||
| Risk Statements | 36/37/38-20 | |||||||||
| Safety Statements | 26-37/39-36 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | FG0700000 | |||||||||
| F | 8 | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 9 | |||||||||
| HS Code | 28432900 | |||||||||
| NFPA 704 |
|
Silver carbonate price More Price(14)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | 85150 | Silver carbonate purum p.a., ≥99.0% (AT) | 534-16-7 | 10G | ₹7503.6 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 85150 | Silver carbonate purum p.a., ≥99.0% (AT) | 534-16-7 | 50G | ₹22455.3 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 363685 | Silver carbonate on Celite® extent of labeling: ~50?wt. % loading | 534-16-7 | 5G | ₹8757.9 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 179647 | Silver carbonate 99% | 534-16-7 | 5G | ₹2586.3 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 363685 | Silver carbonate on Celite® extent of labeling: ~50?wt. % loading | 534-16-7 | 25G | ₹22100.1 | 2022-06-14 | Buy |
Silver carbonate Chemical Properties,Uses,Production
Chemical Properties
Silver carbonate (chemical formula: Ag2CO3) is a light yellow or yellow-green powder, slightly soluble in water, and easily turns dark purple or dark gray under long-term light. After heating, it will decompose to produce silver oxide, which is used to prepare other silver compounds.
Uses
Silver carbonate is a mild oxidizing agent for conversion of alcohols to aldehydes and ketones. It is also used as Biological stain and Koenigs-Knorr glycosylation.
Application
Silver Carbonate on Celite is a reagent used in the synthesis of 3-Oxo-12a-hydroxy-5β-cholanoic Acid which is a keto bile acid derivative.
Preparation
Silver carbonate is prepared by the reaction of sodium carbonate and silver nitrate.
Reaction: Dissolve 53g of sodium carbonate in 600ml of water and slowly add it to a solution of 172g of silver nitrate dissolved in 2L of water (10min). Silver nitrate is in a slight excess. The reaction mixture was vigorously stirred with a mechanical stirrer, and the silver carbonate was filtered off, washed with a small amount of acetone to facilitate drying, and then air-dried. All operations must be carried out in a dark room.
Reactions
Silver carbonate is not a powerful oxidizing agent but it is useful in Organic chemistry. Rapoport et al. were probably the first to use silver carbonate for the oxidation of alcohols to carbonyl derivatives. Rapoport refluxed codeine with silver carbonate in benzene and obtained a 75% yield of codeinone. In later work King et al. oxidized codeine with silver carbonate in refluxing toluene or xylene and obtained an 85% yield of codeinone with a much shorter reaction time.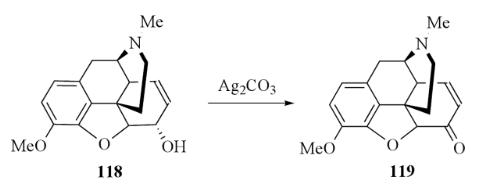
General Description
Odorless yellow to brown solid. Sinks in water.
Reactivity Profile
Silver carbonate is a carbonates salt that has weak oxidizing or reducing powers. It is decomposed by acids with the evolution of carbon dioxide.
Health Hazard
Contact with eyes causes irritation. If continued for a long period, ingestion or inhalation of silver compounds can cause permanent discoloration of the skin (argyria).
Fire Hazard
Behavior in Fire: Decomposes to silver oxide, silver, and carbon dioxide; the reaction is not hazardous.
Silver carbonate Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Evans Fine Chem | +91-9821340302 +91-9821340302 | Maharashtra, India | 286 | 58 | Inquiry |
| Modicon Private Limited | +91-2222825465 +91-2222825464 | Maharashtra, India | 18 | 58 | Inquiry |
| Dhara Industries | +91-9322395199 +91-9322395199 | Mumbai, India | 190 | 58 | Inquiry |
| Micron Platers | +91-2229205235 +91-9821359130 | Maharashtra, India | 20 | 58 | Inquiry |
| S D Fine Chem Limited | +91-9323715856 +91-9323715856 | Maharashtra, India | 368 | 58 | Inquiry |
| ARRAKIS INDUSTRIES LLP | +91 74995 32711 | Maharashtra, India | 1353 | 58 | Inquiry |
| JSK Chemicals | +919879767970 | Gujarat, India | 3756 | 58 | Inquiry |
| FINAR LTD | +91 - 2717 - 616717 | New Delhi, India | 660 | 58 | Inquiry |
| CLEARSYNTH LABS LTD. | +91-22-45045900 | Hyderabad, India | 6257 | 58 | Inquiry |
| A.J Chemicals | 91-9810153283 | New Delhi, India | 6100 | 58 | Inquiry |
| Supplier | Advantage |
|---|---|
| Evans Fine Chem | 58 |
| Modicon Private Limited | 58 |
| Dhara Industries | 58 |
| Micron Platers | 58 |
| S D Fine Chem Limited | 58 |
| ARRAKIS INDUSTRIES LLP | 58 |
| JSK Chemicals | 58 |
| FINAR LTD | 58 |
| CLEARSYNTH LABS LTD. | 58 |
| A.J Chemicals | 58 |
534-16-7(Silver carbonate)Related Search:
1of4
chevron_right




