Silver oxide
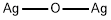
- CAS No.
- 20667-12-3
- Chemical Name:
- Silver oxide
- Synonyms
- SILVER(I) OXIDE;Silver oxid;Silver(I) Oxide 99.5%;Ag2O;Silver oxide;Disilberoxid;ver(I) oxide;disilveroxide;Silver(Ⅰ)Oxide;Oxydisilver(I)
- CBNumber:
- CB3443545
- Molecular Formula:
- Ag2O
- Molecular Weight:
- 231.74
- MOL File:
- 20667-12-3.mol
- MSDS File:
- SDS
- Modify Date:
- 2025/1/27 9:38:02
| Melting point | 300°C (dec.) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Density | 7,143 g/cm3 | ||||||||||||||
| bulk density | 950kg/m3 | ||||||||||||||
| storage temp. | Store below +15°C. | ||||||||||||||
| solubility | insoluble in ethanol; soluble in acid solutions, alkaline solutions | ||||||||||||||
| form | Powder/Solid | ||||||||||||||
| color | Dark-brown | ||||||||||||||
| Specific Gravity | 7.22 | ||||||||||||||
| Water Solubility | slightly soluble | ||||||||||||||
| Sensitive | Light Sensitive | ||||||||||||||
| Crystal Structure | Cu2O type | ||||||||||||||
| crystal system | Cube | ||||||||||||||
| Merck | 14,8521 | ||||||||||||||
| Space group | Pn3m | ||||||||||||||
| Lattice constant |
|
||||||||||||||
| Stability | Stable. Oxidiser. Incompatible with most common metals, ammonia, magnesium, many organic materials. | ||||||||||||||
| InChIKey | KHJDQHIZCZTCAE-UHFFFAOYSA-N | ||||||||||||||
| CAS DataBase Reference | 20667-12-3(CAS DataBase Reference) | ||||||||||||||
| EPA Substance Registry System | Silver(I) oxide (20667-12-3) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS03,GHS05,GHS09 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H271-H318-H410 | |||||||||
| Precautionary statements | P210-P220-P273-P280-P283-P305+P351+P338 | |||||||||
| Hazard Codes | O,Xi,C,N | |||||||||
| Risk Statements | 36/37/38-8-34-50/53-41 | |||||||||
| Safety Statements | 17-26-36-45-36/37/39-61-60-39 | |||||||||
| RIDADR | UN 1479 5.1/PG 2 | |||||||||
| WGK Germany | 2 | |||||||||
| RTECS | VW4900000 | |||||||||
| F | 8 | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 5.1 | |||||||||
| PackingGroup | II | |||||||||
| HS Code | 28432900 | |||||||||
| Toxicity | LD50 orally in rats: 2.82 g/kg (Smyth) | |||||||||
| NFPA 704 |
|
Silver oxide price More Price(26)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | 85260 | Silver(I) oxide purum p.a., ≥99.0% (AT) | 20667-12-3 | 10G | ₹7470.3 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 226831 | Silver(I) oxide ≥99.99% trace metals basis | 20667-12-3 | 1G | ₹2597.4 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 85260 | Silver(I) oxide purum p.a., ≥99.0% (AT) | 20667-12-3 | 50G | ₹27061.8 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 226831 | Silver(I) oxide ≥99.99% trace metals basis | 20667-12-3 | 5G | ₹7492.5 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 221163 | Silver(I) oxide ReagentPlus?, 99% | 20667-12-3 | 10G | ₹3410 | 2022-06-14 | Buy |
Silver oxide Chemical Properties,Uses,Production
Chemical Properties
Silver(I) oxide, Ag2O, is made by action of oxygen under pressure on silver at 300 °C, or by precipitation of a silver salt with carbonate-free alkali metal hydroxide; it is covalent, each silver atom (in solid Ag2O) having two collinear bonds and each oxygen atom four tetrahedral ones; two such interpenetrating lattices constitute the structure.
Physical properties
Brownish-black cubic crystals; density 7.14 g/cm3 at 16°C; begins to decompose around 200°C, decomposition becoming rapid at 250 to 300°C; insoluble in water and ethanol; soluble in acids and alkalis; sparingly soluble in solutions of caustic alkalis; insoluble in alcohol.
Uses
Polishing glass, coloring glass yellow, catalyst, purifying drinking water, lab reagent, carbon dioxide scrubber, and chemical sensors. It is used in the preparation of other silver compounds, and silver-oxide batteries. In organic chemistry, silver oxide finds use as an oxidizing agent for aldehyes to produce carboxylic acids.
Preparation
Silver(I) oxide is precipitated by mixing solutions of silver nitrate and caustic soda: 2AgNO3 + 2NaOH → Ag2O + 2NaNO3 + H2O.
General Description
Odorless brown-black solid. Sinks in water.
Reactivity Profile
Hydrogen sulfide is rapidly oxidized and may ignite in contact with Silver oxide [Bretherick 1979 p. 977]; Mixtures of metal sulfides, gold(III) sulfide, antimony sulfide or mercury (II) sulfide, phosphorus, sulfur, selenium, and selenium disulfide ignite on grinding with the oxide. Ammonia or hydrazine slowly react with Silver oxide forming silver nitride or in the presence of alcohol, silver fulminate may also be produced [Bretherick 1979 p. 203]. Oxidation of magnesium is explosive when warmed with Silver oxide.
Hazard
Fire and explosion risk in contact with organic materials or ammonia.
Health Hazard
Contact with eyes causes mild irritation. If continued for a long period, ingestion or inhalation of silver compounds can cause permanent discoloration of the skin (argyria).
Fire Hazard
Behavior in Fire: Decomposes into metallic silver and oxygen. If large quantities are involved, the oxygen might increase the intensity of the fire.
Safety Profile
A poison by intraperitoneal route. Moderately toxic by ingestion. Flammable by chemical reaction; an oxidizing agent. Explodes in contact with ammonia. Incompatible with CuO, (NH3 + ethanol), (hydrazine + ethanol), CO, HzS, Mg, auric sulfide, Sb sulfide, Hg sulfide, nitroalkanes, Se, S, P, K, Na, NaK, seleninyl chloride. See also SILVER COMPOUNDS.
Purification Methods
Leach the oxide with hot water in a Soxhlet apparatus for several hours to remove any entrained electrolytes. [Glemser & Sauer in Handbook of Preparative Inorganic Chemistry (Ed. Brauer) Academic Press Vol II p 1037 1965.]
Silver oxide Preparation Products And Raw materials
Raw materials
1of2
chevron_rightPreparation Products
1of3
chevron_right| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| GLR Innovations | +91 9891111994 | New Delhi, India | 4535 | 58 | Inquiry |
| Evans Fine Chem | +91-9821340302 +91-9821340302 | Maharashtra, India | 286 | 58 | Inquiry |
| Arora Matthey Limited | +91-9830058614 +91-9830058614 | West Bengal, India | 24 | 58 | Inquiry |
| Dhara Industries | +91-9322395199 +91-9322395199 | Mumbai, India | 190 | 58 | Inquiry |
| ALS INDIA LIFE SCIENCES | +91-8977036379 +91-8977036379 | Hyderabad, India | 1878 | 58 | Inquiry |
| Micron Platers | +91-2229205235 +91-9821359130 | Maharashtra, India | 20 | 58 | Inquiry |
| RYZE CHEMIE | +91-9702966440 +91-9702966440 | Maharashtra, India | 335 | 58 | Inquiry |
| Modicon Private Limited | +91-2222825465 +91-2222825464 | Maharashtra, India | 18 | 58 | Inquiry |
| ARRAKIS INDUSTRIES LLP | +91 74995 32711 | Maharashtra, India | 1353 | 58 | Inquiry |
| JSK Chemicals | +919879767970 | Gujarat, India | 3756 | 58 | Inquiry |
| Supplier | Advantage |
|---|---|
| GLR Innovations | 58 |
| Evans Fine Chem | 58 |
| Arora Matthey Limited | 58 |
| Dhara Industries | 58 |
| ALS INDIA LIFE SCIENCES | 58 |
| Micron Platers | 58 |
| RYZE CHEMIE | 58 |
| Modicon Private Limited | 58 |
| ARRAKIS INDUSTRIES LLP | 58 |
| JSK Chemicals | 58 |
20667-12-3(Silver oxide)Related Search:
1of4
chevron_right




