Ferric oxide
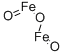
- CAS No.
- 1309-37-1
- Chemical Name:
- Ferric oxide
- Synonyms
- Diiron Trioxide;Pigment red 101;Red Ferric Oxide;supra;ochre;deanox;raddle;rubigo;ferrugo;ironred
- CBNumber:
- CB3115644
- Molecular Formula:
- Fe2O3
- Molecular Weight:
- 159.69
- MOL File:
- 1309-37-1.mol
- MSDS File:
- SDS
- Modify Date:
- 2024/6/29 22:56:24
| Melting point | 1538°C |
|---|---|
| Density | 5.24 |
| Flash point | >230 °F |
| storage temp. | 2-8°C |
| solubility | It is soluble In Warm Hydrochloric Acid, Slightly Soluble in Sulfuric Acid. |
| form | pieces |
| color | black |
| Specific Gravity | 5.1~5.2 |
| PH | 3.7±0.3 |
| Water Solubility | INSOLUBLE |
| Merck | 14,4028 |
| Exposure limits |
ACGIH: TWA 5 mg/m3 OSHA: TWA 10 mg/m3; TWA 15 mg/m3; TWA 5 mg/m3 NIOSH: IDLH 2500 mg/m3; TWA 5 mg/m3 |
| Stability | Stable. |
| CAS DataBase Reference | 1309-37-1(CAS DataBase Reference) |
| IARC | 3 (Vol. 1, Sup 7) 1987 |
| NIST Chemistry Reference | Iron(iii) oxide(1309-37-1) |
| EPA Substance Registry System | Ferric oxide (1309-37-1) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS02,GHS05 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H225-H318 | |||||||||
| Precautionary statements | P210-P280-P305+P351+P338+P310 | |||||||||
| Hazard Codes | Xi | |||||||||
| Risk Statements | 36/37/38 | |||||||||
| Safety Statements | 26 | |||||||||
| OEB | B | |||||||||
| OEL | TWA: 5 mg/m3 | |||||||||
| WGK Germany | - | |||||||||
| RTECS | NO7400000 | |||||||||
| TSCA | Yes | |||||||||
| HS Code | 28211000 | |||||||||
| IDLA | 2,500 mg Fe/m3 | |||||||||
| NFPA 704 |
|
Ferric oxide price More Price(59)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | 747432 | Iron oxide(II,III) magnetic nanoparticles solution 30?nm avg. part. size (TEM), biotin functionalized, 1?mg/mL Fe in H2O, dispersion | 1309-37-1 | 1ML | ₹34925.8 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 747335 | Iron oxide(II,III) magnetic nanoparticles solution 30?nm avg. part. size (TEM), carboxylic acid functionalized, dispersion | 1309-37-1 | 2ML | ₹31042.2 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 720712 | Iron(III) oxide, dispersion nanoparticles, ≤110?nm particle size, 15?wt. % in ethanol | 1309-37-1 | 100G | ₹20856.83 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 544884 | Iron(III) oxide nanopowder, <50?nm particle size (BET) | 1309-37-1 | 5G | ₹3929.48 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 544884 | Iron(III) oxide nanopowder, <50?nm particle size (BET) | 1309-37-1 | 25G | ₹13098.25 | 2022-06-14 | Buy |
Ferric oxide Chemical Properties,Uses,Production
Description
Iron oxides are produced synthetically and consist essentially of anhydrous and/or hydrated iron oxides. The range of hues includesyellows, reds, browns and blacks. Food quality iron oxides are primarily distinguished from technical grades by the comparatively low levels of contamination by other metals. This is achieved by the selection and control of the source of iron and/or by the extent of chemical purification during the manufacturing process. Iron oxides have been used to color confectionery, fillings and decorations for pastry products, cheese products, fish paste, pet foods, cosmetics and pharmaceutical products.
Chemical Properties
Hematite is a noncombustible, black to black red or brick-red mineral (iron ore) composed mainly of ferric oxide, Fe2O3. Ferric oxide
Occurrence
Iron(III) oxide occurs in nature as the mineral hematite. It is the principal ore of iron from which the metal and its alloys are produced. Also, this oxide occurs in the mineral, limonite, 2Fe2O3?3H2O. An important application of this compound involves producing red, orange, and yellow pigments. Other applications are in coatings for metals, steel and rubber; in ceramics; and as a catalyst for oxidation reactions.
Uses
Ferric Oxide is a nutrient and dietary supplement that is a source of iron.
Preparation
Iron(III) oxide is prepared as a reddish-brown hydrated precipitate by treating an aqueous solution of an iron(III) salt with caustic soda:
2FeCl3 + 6NaOH → Fe2O3?3H2O + 6NaCl
It also is obtained by thermal decomposition of iron(II) sulfate or the brown oxide hydroxide:
2FeSO4 → Fe2O3 + SO2 + SO3
2FeO(OH) → Fe2O3 + H2O
The oxide is prepared in industrial scale by first precipitating iron(II) hydroxide Fe(OH)2 by treating aqueous solutions of iron(II) sulfate and caustic soda. The Fe(OH)2 is then oxidized to iron(III) hydroxide by aeration. The latter is dehydrated by heating:
Fe2+ (aq) + OHˉ (aq) → Fe(OH)2(s) → 2Fe(OH)3 → Fe2O3 + 3H2O
It also is produced by ignition of iron(III) oxalate and iron carbonyls:
2Fe2(C2O4)3 +3O2 → 2Fe2O3 + 12CO
.
Definition
A high-grade red pigment used as a polishing agent for glass, jewelry, etc. (2) A cosmetic prepared from dried flowers of the saf- flower.
Hazard
Pneumoconiosis. Questionable carcinogen.
Potential Exposure
Hematite; as an iron ore composed mainly of ferric oxide, is a major source of iron and is used as a pigment for rubber, paints, paper, linoleum, ceramics, dental restoratives; and as a polishing agent for glass and pre cious metals. It is also used in electrical resistors, semiconduc tors, magnets, and as a catalyst. Human exposure to hematite from underground hematite mining is principally through inhalation and/or ingestion of dusts. No estimates are available concerning the number of underground miners exposed.
Carcinogenicity
Welders are typically exposed to a complex mixture of dust and fume of metallic oxides, as well as irritant gases, and are subject to mixeddust pneumoconiosis with possible loss of pulmonary function; this should not be confused with benign pneumoconiosis caused by iron oxide.1 Although an increased incidence of lung cancer has been observed among hematite miners exposed to iron oxide, presumably owing to concomitant radon gas exposure, there is no evidence that iron oxide alone is carcinogenic to man or animals.6
Incompatibilities
Contact with hydrogen peroxide, ethyl ene oxide, calcium hypochlorite will cause explosion. Violent reaction with powdered aluminum; hydrazine, hydrogen trisulfide.
Ferric oxide Preparation Products And Raw materials
Raw materials
1of5
chevron_rightPreparation Products
1of2
chevron_right| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| VAYUNANDAN IMPEX | +919836761502 | Rajasthan, India | 28 | 58 | Inquiry |
| UNILOSA INTERNATINAL PRIVATE LIMITED | +91-9999069917 +91-9999069917 | New Delhi, India | 152 | 58 | Inquiry |
| Parshwanath Group of Indusries | +91-9979874744 +91-9825244644 | Gujarat, India | 211 | 58 | Inquiry |
| Vidhi Specialty Food Ingredients Ltd | +91-2223514349 +91-2261406666 | Maharashtra, India | 17 | 58 | Inquiry |
| Kings International | +91-228048077732 +91-228048077732 | Maharashtra, India | 8 | 58 | Inquiry |
| Ecosense Labs. Pvt. Ltd. | +9102222009647 | Mumbai, India | 16 | 58 | Inquiry |
| Vega Chemicals Pvt Ltd | +91-8888857715 +91-8888857715 | Maharashtra, India | 2 | 58 | Inquiry |
| Krishna Industries | +91-7922822554 +91-7922822554 | Gujarat, India | 235 | 58 | Inquiry |
| Nitika Pharmaceutical Specialities Pvt Ltd | +91-7122641001 +91-8956932443 | Maharashtra, India | 36 | 58 | Inquiry |
| Shlok Enterprise | +91-2222700992 +91-9833968666 | Maharashtra, India | 9 | 58 | Inquiry |
Related articles
- Ferric oxide: Polarity and Application
- Fe2O3 is an ionic compound. The electronegativity difference between iron and oxygen atoms is 1.6, which corresponds to the el....
- Jan 4,2024
1309-37-1(Ferric oxide )Related Search:
1of4
chevron_right




