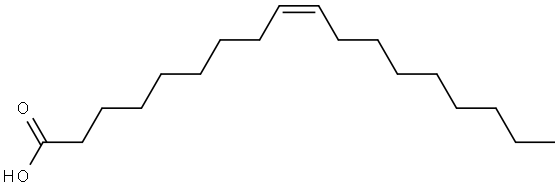
Oleic acid
- CAS No.
- 112-80-1
- Chemical Name:
- Oleic acid
- Synonyms
- Octadecenoic acid;METHANONE;FORMALDEHYDE SOLUTION;cis-9-Octadecenoic acid;D100;9-cis-Octadecenoicacid;Oleic acid, AR;Oleinic acid;cis-Oleic Acid;Oleic Acid - CAS 112-80-1 - Calbiochem
- CBNumber:
- CB7228241
- Molecular Formula:
- C18H34O2
- Molecular Weight:
- 282.46
- MOL File:
- 112-80-1.mol
- MSDS File:
- SDS
- Modify Date:
- 2024/8/21 22:41:43
| Melting point | 13-14 °C(lit.) |
|---|---|
| Boiling point | 360 °C |
| Density | 0.89 g/mL at 25 °C(lit.) |
| vapor density | 1.03 (vs air) |
| vapor pressure | 52 mm Hg ( 37 °C) |
| FEMA | 2815 | OLEIC ACID |
| refractive index |
n |
| Flash point | 133 °F |
| storage temp. | -20°C |
| solubility | Miscible with ethanol, ether, acetone, chloroform, dimethyl formamide and dimethyl sulfoxide. |
| form | Liquid |
| pka | pKa 5.35(H2O,t =25) (Uncertain) |
| Specific Gravity | 0.892 (20/4℃) |
| color | Colorless to pale yellow |
| Odor | Peculiar Lard-Like |
| Odor Type | fatty |
| Water Solubility | negligible |
| Sensitive | Air Sensitive |
| JECFA Number | 333 |
| Merck | 14,6828 |
| BRN | 1726542 |
| Hydrophilic-Lipophilic Balance (HLB) | 1 |
| Dielectric constant | 2.5(20℃) |
| Stability | Stable. Combustible. Incompatible with strong oxidizing agents, aluminium. |
| InChIKey | ZQPPMHVWECSIRJ-KTKRTIGZSA-N |
| LogP | 7.698 (est) |
| CAS DataBase Reference | 112-80-1(CAS DataBase Reference) |
| NIST Chemistry Reference | 9-Octadecenoic acid (Z)-(112-80-1) |
| EPA Substance Registry System | Oleic acid (112-80-1) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H320-H319-H315 | |||||||||
| Precautionary statements | P280a-P305+P351+P338-P321-P332+P313-P337+P313-P264-P280-P302+P352+P332+P313+P362+P364-P305+P351+P338+P337+P313 | |||||||||
| Hazard Codes | T,Xi | |||||||||
| Risk Statements | 23/24/25-34-40-43-36/37/38-38 | |||||||||
| Safety Statements | 36/37-37/39-26-36-36/37/39 | |||||||||
| RIDADR | UN 1198 3/PG 3 | |||||||||
| WGK Germany | 2 | |||||||||
| RTECS | LP8925000 | |||||||||
| F | 10 | |||||||||
| Autoignition Temperature | 363°C | |||||||||
| TSCA | Yes | |||||||||
| HS Code | 29161500 | |||||||||
| Toxicity | LD50 i.v. in mice: 230±18 mg/kg (Or, Wretlind) | |||||||||
| NFPA 704 |
|
Oleic acid price More Price(34)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | W281506 | Oleic acid natural, FCC | 112-80-1 | 1SAMPLE-K | ₹5196 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | W281506 | Oleic acid natural, FCC | 112-80-1 | 1KG | ₹8086.28 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | W281506 | Oleic acid natural, FCC | 112-80-1 | 4KG | ₹17114.33 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | W281506 | Oleic acid natural, FCC | 112-80-1 | 10KG | ₹33557.5 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | W281506 | Oleic acid natural, FCC | 112-80-1 | 20KG | ₹42336.58 | 2022-06-14 | Buy |
Oleic acid Chemical Properties,Uses,Production
Chemical Properties
Oleic acid, C17H33COOH, also known as red oil, elaine oil, and octadecenoic acid, is a yellowish unsaturated fatty acid with an aroma similar to lard. Oleic acid consists chiefly of (Ζ)-9-octadecenoic acid together with varying amounts of saturated and other unsaturated acids. It is insoluble in water, but soluble in most organic solvents. Oleic acid is the main component in cooking and olive oils.It is used for making aluminum oleate, which thickens lubricating oil, and in the preparation of soaps and cosmetics.
Occurrence
Reported found in apple, banana, cranberry, guava, grapes, melon, papaya, ginger, hop oil, ginger, beef fat, beer, rum, whiskies, cider, sherry, tea, goat milk, butterfat, celery, cheese, blue cheese, munster cheese, other cheeses, cognac, country cured ham, pork fat, potato, raspberry oil, tomato, peanut oil, coconut meat, avocado, mushroom, fenugreek, tamarind, kelp, cardamom, rice, dill seed, sake, buckwheat, malt, wort, roasted chicory root and cape gooseberry.
Uses
Oleic acid is a monounsaturated omega-9 fatty acid. Oleic Acid is obtained by the hydrolysis of various animal and vegetable fats and oils. Oleic Acid is used as an emulsifying or solubilizing agent i n aerosol products.
Production Methods
Oleic acid is obtained by the hydrolysis of various animal and vegetable fats or oils, such as olive oil, followed by separation of the liquid acids. It consists chiefly of (Ζ)-9-octadecenoic acid. Oleic acid that is to be used systemically should be prepared from edible sources.
Definition
ChEBI: Oleic acid is an octadec-9-enoic acid in which the double bond at C-9 has Z (cis) stereochemistry. It has a role as an EC 3.1.1.1 (carboxylesterase) inhibitor, an Escherichia coli metabolite, a plant metabolite, a Daphnia galeata metabolite, a solvent, an antioxidant and a mouse metabolite. It is a conjugate acid of an oleate. It derives from a hydride of a cis-octadec-9-ene.
General Description
Colorless to pale yellow liquid with a mild odor. Floats on water.
Air & Water Reactions
Keep cis-9-Octadecenoic acid well closed; protect cis-9-Octadecenoic acid from air and light. . May form peroxides upon exposure to air. This is taken to account for an explosion that occurred, by the mixing of the acid with aluminum, [J. Chem. Educ., 1956, 36, 308]. Water Insoluble.
Health Hazard
Industrial use of compound involves no known hazards. Ingestion causes mild irritation of mouth and stomach. Contact with eyes or skin causes mild irritation.
Fire Hazard
cis-9-Octadecenoic acid is combustible.
Pharmaceutical Applications
Oleic acid is used as an emulsifying agent in foods and topical
pharmaceutical formulations. It has also been used as a penetration enhancer in transdermal formulations,to improve the bioavailability
of poorly water-soluble drugs in tablet formulations,
and as part of a vehicle in soft gelatin capsules, in topical
microemulsion formulations,in oral self-emulsifying drug
delivery systems,in oral mucoadhesive patches,and in a
metered dose inhaler.Oleic acid was shown to be an important
factor in the hypoglycemic effect produced by multiple emulsions
containing insulin intended for intestinal delivery of insulin.
The phase behavior of sonicated dispersions of oleic acid has
been described,and mechanisms for the topical penetrationenhancing
actions of oleic acid have been presented.
Oleic acid has been reported to act as an ileal ‘brake’ that slows
down the transit of luminal contents through the distal portion of
the small bowel.
Oleic acid labeled with 131I and 3H is used in medical imaging.
Safety Profile
Poison by intravenous route. Mildly toxic by ingestion. Mutation data reported. A human skin and eye irritant. Questionable carcinogen with experimental tumorigenic data. Combustible when exposed to heat or flame. To fight fire, use CO2, dry chemical. The peroxidzed acid explodes on contact with aluminum. Potentially dangerous reaction with perchloric acid + heat. When heated to decomposition it emits acrid smoke and irritating fumes.
Safety
Oleic acid is used in oral and topical pharmaceutical formulations.
In vitro tests have shown that oleic acid causes rupture of red
blood cells (hemolysis), and intravenous injection or ingestion of a
large quantity of oleic acid can therefore be harmful. The effects of
oleic acid on alveolar and buccal epithelial cells in vitro have
also been studied; the in vitro and in vivo effects of oleic acid on rat
skin have been reported. Oleic acid is a moderate skin irritant; it
should not be used in eye preparations.
An acceptable daily intake for the calcium, sodium, and
potassium salts of oleic acid was not specified by the WHO since
the total daily intake of these materials in foods was such that they
did not pose a hazard to health.
LD50 (mouse, IV): 0.23 g/kg
LD50 (rat, IV): 2.4 mg/kg
LD50 (rat, oral): 74 g/kg
Carcinogenicity
Some recent studies suggested that oleic acid may decrease the incidence of mammary gland tumors of some rodent species. In a reviewof several fatty acids, Ip concludes that there is little evidence for the protective effect of oleic acid on the development of cancer.
storage
On exposure to air, oleic acid gradually absorbs oxygen, darkens in
color, and develops a more pronounced odor. At atmospheric
pressure, it decomposes when heated at 80–100°C.
Oleic acid should be stored in a well-filled, well-closed container,
protected from light, in a cool, dry place.
Purification Methods
Purify the acid by fractional crystallisation from its melt, followed by molecular distillation at 10 -3mm, or by conversion to its methyl ester, the free acid can be crystallised from acetone at -40o to -45o (12mL/g). For purification by the use of lead and lithium salts, see Keffler and McLean [J Soc Chem Ind (London) 54 176T 1935]. Purification based on direct crystallisation from acetone is described by Brown and Shinowara [J Am Chem Soc 59 6 1937, pK White J Am Chem Soc 72 1857 1950]. [Beilstein 2 H 463, 2 I 198, 2 II 429, 2 III 1387, 2 IV 1641.]
Incompatibilities
Incompatible with aluminum, calcium, heavy metals, iodine solutions, perchloric acid, and oxidizing agents. Oleic acid reacts with alkalis to form soaps.
Regulatory Status
GRAS listed. Included in the FDA Inactive Ingredients Database (inhalation and nasal aerosols, tablets, topical and transdermal preparations). Included in nonparenteral medicines (metered dose inhalers; oral capsules; oral prolonged release granules; topical creams and gels) licensed in the UK. Included in the Canadian List of Acceptable Non-medicinal Ingredients.
Oleic acid Preparation Products And Raw materials
Raw materials
1of5
chevron_rightPreparation Products
1of8
chevron_right| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Otto Chemie Pvt. Ltd. | +91 9820041841 | Mumbai, India | 32 | 58 | Inquiry |
| AnalyticsStanza Inc | +91-7032031309 +91-7032031309 | Hyderabad, India | 227 | 58 | Inquiry |
| UNILOSA INTERNATINAL PRIVATE LIMITED | +91-9999069917 +91-9999069917 | New Delhi, India | 152 | 58 | Inquiry |
| Venus Ethoxyethers Pvt Ltd | +91-8322383333 +91-8669968881 | GOA, India | 19 | 58 | Inquiry |
| Agilenobel | +91-8090295395 +91-8800293647 | Haryana, India | 50 | 58 | Inquiry |
| Neugen Labs | +91-8133254502 +91-8133254502 | Karnataka, India | 195 | 58 | Inquiry |
| Esteem Industries Pvt. Ltd. | +91-8326613321 +91-8669968881 | Mumbai, India | 85 | 58 | Inquiry |
| Rossari Biotech Ltd. | +91-2261233800 +91-2261233800 | Mumbai, India | 57 | 58 | Inquiry |
| Progress Life Sciences Pvt Ltd | +91-2265101706 +91-2249781414 | Maharashtra, India | 38 | 58 | Inquiry |
| S D Fine Chem Limited | +91-9323715856 +91-9323715856 | Maharashtra, India | 368 | 58 | Inquiry |
| Supplier | Advantage |
|---|---|
| Otto Chemie Pvt. Ltd. | 58 |
| AnalyticsStanza Inc | 58 |
| UNILOSA INTERNATINAL PRIVATE LIMITED | 58 |
| Venus Ethoxyethers Pvt Ltd | 58 |
| Agilenobel | 58 |
| Neugen Labs | 58 |
| Esteem Industries Pvt. Ltd. | 58 |
| Rossari Biotech Ltd. | 58 |
| Progress Life Sciences Pvt Ltd | 58 |
| S D Fine Chem Limited | 58 |
Related articles
- A substance rich in many foods - oleic acid
- Oleic acid is the most widely distributed natural fatty acid and is present in practically all lipids.
- Nov 21,2023
- The Uses of Oleic Acid
- The passage introduces the uses of Oleic Acid.
- Nov 24,2022
- Preparation and use of oleic acid
- This article is about the physical and chemical properties of oleic acid and the description of its preparation and use
- Feb 18,2022
Related Qustion
- Q:Is Oleic acid a saturated or unsaturated fatty acid? And what does it do for the human body?
- A:Oleic acid is (OA) an unsaturated fatty acid that occurs naturally in animal and vegetable oils.
- May 6,2024





