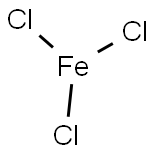
Ferric chloride
- CAS No.
- 7705-08-0
- Chemical Name:
- Ferric chloride
- Synonyms
- FeCl3;IRON(III) CHLORIDE;IRON CHLORIDE;Ferric chloride anhydrous;FERRIC CHLORIDE SOLUTION;DB03;FERRIC CHLORID;Iron perchloride;IRON TRICHLORIDE;Iron(Ⅲ) chloride
- CBNumber:
- CB5444364
- Molecular Formula:
- Cl3Fe
- Molecular Weight:
- 162.2
- MOL File:
- 7705-08-0.mol
- MSDS File:
- SDS
- Modify Date:
- 2024/7/30 17:43:28
| Melting point | 304 °C(lit.) |
|---|---|
| Boiling point | 316 °C |
| Density | 2,804 g/cm3 |
| vapor density | 5.61 (vs air) |
| vapor pressure | 1 mm Hg ( 194 °C) |
| refractive index | n20/D1.414 |
| Flash point | 316°C |
| storage temp. | Store below +30°C. |
| solubility | H2O: soluble |
| form | powder |
| color | Yellow |
| Specific Gravity | 2.804 |
| PH | 1 (200g/l, H2O, 20℃) |
| Water Solubility | 920 g/L (20 ºC) |
| Sensitive | Hygroscopic |
| Merck | 14,4019 |
| Exposure limits |
ACGIH: TWA 1 mg/m3 NIOSH: TWA 1 mg/m3 |
| Stability | Stable. Very sensitive to moisture. Incompatible with strong oxidizing agents; forms explosive mixtures with sodium, potassium. Hygroscopic. |
| CAS DataBase Reference | 7705-08-0(CAS DataBase Reference) |
| NIST Chemistry Reference | Ferric chloride(7705-08-0) |
| EPA Substance Registry System | Ferric chloride (7705-08-0) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |     GHS02,GHS05,GHS07,GHS08 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H225-H290-H302-H318-H335-H351 | |||||||||
| Precautionary statements | P201-P210-P233-P261-P280-P305+P351+P338+P310 | |||||||||
| Hazard Codes | C,Xn,Xi | |||||||||
| Risk Statements | 41-38-22-34-37/38-10-36 | |||||||||
| Safety Statements | 26-39-45-36/37/39 | |||||||||
| RIDADR | UN 2582 8/PG 3 | |||||||||
| WGK Germany | 1 | |||||||||
| RTECS | LJ9100000 | |||||||||
| TSCA | Yes | |||||||||
| HS Code | 2827 39 20 | |||||||||
| HazardClass | 8 | |||||||||
| PackingGroup | III | |||||||||
| Toxicity | LD50 orally in Rabbit: 316 mg/kg | |||||||||
| NFPA 704 |
|
Ferric chloride price More Price(29)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | 8.03945 | Iron(III) chloride anhydrous for synthesis | 7705-08-0 | 1KG | ₹8280 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 908908 | Iron(III) chloride free-flowing, Redi-Dri?, reagent grade, 97% | 7705-08-0 | 100G | ₹5206.83 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 8.03945 | Iron(III) chloride anhydrous for synthesis | 7705-08-0 | 500G | ₹10040 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 809330 | Fe/ppm Pd for nitro group reductions | 7705-08-0 | 500MG | ₹4384.13 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 8.03945 | Iron(III) chloride anhydrous for synthesis | 7705-08-0 | 60KG | ₹86010 | 2022-06-14 | Buy |
Ferric chloride Chemical Properties,Uses,Production
Description
Ferric chloride (iron(IH)chloride, FeCl3, CAS No. 7705-08-0) may be prepared from iron and chlorine or from ferric oxide and hydrogen chloride. The pure material occurs as hydroscopic, hexagonal, dark crystals. Ferric chloride hexahydrate (iron(III)chloride hexahydrate, FeCl3*6H2O, CAS No. 10025-77-1) is readily formed when ferric chloride is exposed to moisture.
Chemical Properties
Ferric chloride,FeCl3, is a brown crystalline solid and is soluble in water,alcohol,and glycerol. It is also known as anhydrous ferric chloride,ferric trichloride, Flores martis,and iron chloride. Ferric chloride is used as a coagulant for sewage and industrial wastes, as an oxidizing and chlorinating agent,as a disinfectant, in copper etching, and as amordant. In addition, this compound is employed in the ferric chloride test,which is used to assess the relative corrosion resistance of stainless and nickel-base alloys. The ferric chloride test has been shown to be an appropriate measure of the suitability of such alloys for service in paper mill bleach plants and seawater.
Uses
Ferric Chloride is a nutrient and dietary supplement that serves as a source of iron.
General Description
Ferric chloride is an orange to brown-black solid. Ferric chloride is slightly soluble in water. Ferric chloride is noncombustible. When wet Ferric chloride is corrosive to aluminum and most metals. Pick up and remove spilled solid before adding water. Ferric chloride is used to treat sewage, industrial waste, to purify water, as an etching agent for engraving circuit boards, and in the manufacture of other chemicals.
Air & Water Reactions
Very hygroscopic. Slightly water soluble, where a 0.1M solution has a pH of 2.0.
Reactivity Profile
Alkali metal hydroxides, acids, anhydrous chlorides of iron, tin, and aluminum, pure oxides of iron and aluminum, and metallic potassium are some of the catalysts that may cause ethylene oxide to rearrange and polymerize, liberating heat, [J. Soc. Chem. Ind. 68:179(1949)]. Explosions occur , although infrequently, from the combination of ethylene oxide and alcohols or mercaptans, [Chem. Eng. News 20:1318(1942)]. Allyl chloride may polymerize violently under conditions involving an acid catalyst, such as sulfuric acid, Ferric chloride, aluminum chloride, Lewis acids, and Ziegler type catalysts (initiators), [Ventrone (1971)].
Hazard
Toxic by ingestion, strong irritant to skin and tissue.
Health Hazard
Inhalation of dust may irritate nose and throat. Ingestion causes irritation of mouth and stomach. Dust irritates eyes. Prolonged contact with skin causes irritation and burns.
Fire Hazard
Special Hazards of Combustion Products: Irritating hydrogen chloride fumes may form in fire.
Industrial uses
Ferric chloride (FeCl3) is obtained by an iron chlorination method at a temperature of 600–700 °C. Very limited data are available on the use of ferric chloride in the mineral processing industry. Ferric chloride has a depressing effect on barite and can be used in barite–celestite separation. It was also evaluated as a depressant during niobium– zirconium separation. In general, ferric and ferrous compounds are not selective depressants and in many cases are detrimental for flotation of oxidic and industrial minerals as in the case of anionic flotation, fatty acid, iron complexes or oleate iron complexes.
Safety Profile
Poison by ingestion and intravenous routes. Experimental reproductive effects. Corrosive. Probably an eye, skin, and mucous membrane irritant. Mutation data reported. Reacts with water to produce toxic and corrosive fumes. Catalyzes potentially explosive polymerization of ethylene oxide, chlorine + monomers (e.g., styrene). Forms shock sensitive explosive mixtures with some metals (e.g., potassium, sodium). Violent reaction with all$ chloride. When heated to decomposition it emits highly toxic fumes of HCl.
Potential Exposure
Iron chloride is used to treat sewage and industrial waste. It is also used as an etchant for photo engraving and rotogravure; in textiles; photography; as a disinfectant; as a feed additive.
Shipping
UN1773 Ferric chloride, anhydrous, Hazard class: 8; Labels: 8-Corrosive material. UN2582 Ferric chlo ride, solution, Hazard class: 8; Labels: 8-Corrosive material
Purification Methods
Sublime it at 200o in an atmosphere of chlorine. It is an “iron-black” coloured powder with green irridescence. Store it in a weighing bottle inside a desiccator as it absorbs moisture from air to form the yellow hexahydrate (see next entry). [Tarr Inorg Synth III 191 1950, Pray Inorg Synth V 153 1957, Epperson Inorg Synth VII 163 1963.]
Incompatibilities
Aqueous solutions are a strong acid. Violent reaction with bases, allyl chloride; sulfuric acid; water. Shock- and friction-sensitive explosive material forms with potassium, sodium and other active metals. Attacks metals when wet.
Waste Disposal
Neutralize with lime or soda ash and bury in an approved landfill.
Ferric chloride Preparation Products And Raw materials
Raw materials
1of2
chevron_rightPreparation Products
1of8
chevron_right| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| SUKHA CHEMICAL INDUSTRIES | +91-9638875444 +91-9638875444 | Gujarat, India | 26 | 58 | Inquiry |
| UNILOSA INTERNATINAL PRIVATE LIMITED | +91-9999069917 +91-9999069917 | New Delhi, India | 152 | 58 | Inquiry |
| JSK Chemicals | +919879767970 | Gujarat, India | 3756 | 58 | Inquiry |
| Annexe Chem Pvt Ltd | +91-9724624061 +91-9898722162 | Gujarat, India | 57 | 58 | Inquiry |
| Speqtus Industries | +91-9752001547 +91-9988988827 | Gujarat, India | 4 | 58 | Inquiry |
| DCW Limited | +91-2222871916 +91-2222871914 | Maharashtra, India | 8 | 58 | Inquiry |
| Real Metal Chem Pvt Ltd | +91 9825454419 +91 9825454622 | Gujarat, India | 4 | 58 | Inquiry |
| Shriji Chemicals | +91-9822328437 +91-9822328437 | Maharashtra, India | 14 | 58 | Inquiry |
| Innovacorp India Pvt Ltd | +91-9312871070 +91-9911981992 | New Delhi, India | 23 | 58 | Inquiry |
| Khushi Chemicals Pvt Ltd | +91-8000009797 +91-7818888999 | Gujarat, India | 6 | 58 | Inquiry |
| Supplier | Advantage |
|---|---|
| SUKHA CHEMICAL INDUSTRIES | 58 |
| UNILOSA INTERNATINAL PRIVATE LIMITED | 58 |
| JSK Chemicals | 58 |
| Annexe Chem Pvt Ltd | 58 |
| Speqtus Industries | 58 |
| DCW Limited | 58 |
| Real Metal Chem Pvt Ltd | 58 |
| Shriji Chemicals | 58 |
| Innovacorp India Pvt Ltd | 58 |
| Khushi Chemicals Pvt Ltd | 58 |
Related articles
- What is the charge of the metal ion in FeCl3?
- Ferric chloride is named as iron chloride. It is a chemical compound with a chemical formula consisting of FeCl3. The charge ....
- Feb 19,2024
- Application of Ferric trichloride
- Ferric trichloride is an important chemical raw material, because it has trivalent iron ions, so ferric trichloride has good o....
- Jan 13,2022
7705-08-0(Ferric chloride)Related Search:
1of4
chevron_right




