Trichlorosilane
- CAS No.
- 10025-78-2
- Chemical Name:
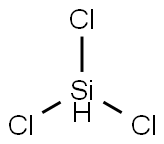
- Trichlorosilane
- Synonyms
- trichlorsilan;silicon chloroform;orosiL;TrichL;silanea-19;SiHCl3(TCS) ;triclorosilano;Trichorosilane;TRICHLOROSILANE;trichloorsilaan
- CBNumber:
- CB4852559
- Molecular Formula:
- Cl3HSi
- Molecular Weight:
- 135.45
- MOL File:
- 10025-78-2.mol
- MSDS File:
- SDS
- Modify Date:
- 2023/9/4 14:20:59
| Melting point | -127 °C |
|---|---|
| Boiling point | 32-34 °C(lit.) |
| Density | 1.342 g/mL at 25 °C(lit.) |
| vapor density | 1 (vs air) |
| vapor pressure | 9.75 psi ( 20 °C) |
| refractive index | 1.4-1.402 |
| Flash point | 7 °F |
| storage temp. | Refrigerator |
| solubility | Soluble in benzene, ether, heptane, chloroform and carbon tetrachloride. |
| form | Liquid |
| Specific Gravity | 1.342 |
| color | Colorless |
| Odor | Sharp, choking, like hydrochloric acid. |
| explosive limit | 70% |
| Water Solubility | decomposes |
| Sensitive | Air & Moisture Sensitive |
| Hydrolytic Sensitivity | 8: reacts rapidly with moisture, water, protic solvents |
| Merck | 14,9646 |
| Stability | Stable, but extremely flammable. Pyrophoric - spontaneously ignites in air. Explosion hazard in air - note wide explosion limits. Reacts violently with water. Incompatible with moisture, acids, bases, strong oxidizing agents, alcohols, amines. |
| InChIKey | ZDHXKXAHOVTTAH-UHFFFAOYSA-N |
| CAS DataBase Reference | 10025-78-2(CAS DataBase Reference) |
| NIST Chemistry Reference | Trichlorosilane(10025-78-2) |
| EPA Substance Registry System | Trichlorosilane (10025-78-2) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS02,GHS05,GHS06 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H224-H250-H302-H314-H331-H335 | |||||||||
| Precautionary statements | P210-P233-P280-P303+P361+P353-P305+P351+P338-P403+P233 | |||||||||
| Hazard Codes | F+,C | |||||||||
| Risk Statements | 12-14-17-20/22-29-35 | |||||||||
| Safety Statements | 16-26-36/37/39-43-45-7/9-43A | |||||||||
| RIDADR | UN 1295 4.3/PG 1 | |||||||||
| WGK Germany | 1 | |||||||||
| RTECS | VV5950000 | |||||||||
| F | 4.5-21-31 | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 4.3 | |||||||||
| PackingGroup | I | |||||||||
| HS Code | 28530090 | |||||||||
| Toxicity | LD50 orally in rats: 1.03 g/kg (Smyth) | |||||||||
| NFPA 704 |
|
Trichlorosilane price More Price(3)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| ALFA India | ALF-014078-36 | Trichlorosilane, 98% | 10025-78-2 | 500g | ₹15432 | 2022-05-26 | Buy |
| ALFA India | ALF-014078-22 | Trichlorosilane, 98% | 10025-78-2 | 100g | ₹6481 | 2022-05-26 | Buy |
| ALFA India | ALF-014078-14 | Trichlorosilane, 98% | 10025-78-2 | 25g | ₹1615 | 2022-05-26 | Buy |
Trichlorosilane Chemical Properties,Uses,Production
Chemical Properties
Clear liquid with acrid odor of hydrogen chloride. soluble in carbon disulfide, carbon tetrachloride, chloroform, benzene, etc.
Uses
Trichlorosilane is used in process of hydrosilylation. It is also used for reductive hydrazination which is a high yielding method for preparation of 1,1-disubstituted hydrazines.
General Description
A colorless fuming liquid with a pungent odor. Flash point 7°F. Vapor and liquid cause burns. More dense than water. Vapors are heavier than air.
Air & Water Reactions
Highly flammable. Ignites spontaneously in air [NFPA, 1991]. Reacts violently with water, steam, moisture in air to generate heat and flammable (H2) and corrosive (HCl) gases. [Handling Chemicals Safely 1980. p. 924].
Reactivity Profile
Trichlorosilane reacts with alcohols, acetone, light metals with generation of heat and combustible (H2) and corrosive (HCl) gases [Handling Chemicals Safely 1980. p. 924].
Health Hazard
Inhalation causes severe irritation of respiratory system. Liquid causes severe burns of eyes and skin. Ingestion causes severe burns of mouth and stomach.
Safety Profile
Moderately toxic by ingestion and inhalation. A corrosive irritant to skin, eyes, and mucous membranes. A very dangerous fire hazard when exposed to heat, flame, or by chemical reaction. May be ignited by spark or impact. Spontaneously flammable in air. Explosive reaction with acetonitrile + diphenyl sulfoxide. Will react with water or steam to produce heat and toxic and corrosive fumes. Can react vigorously with oxidizing materials. To fight fire, use CO2, dry chemical. When heated to decomposition it emits toxic fumes of Cl-. See also CHLOROSILANES.
Trichlorosilane Preparation Products And Raw materials
Raw materials
Preparation Products
1of4
chevron_right| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Alfa Aesar | 1 800 209 7001 | Maharashtra, India | 6913 | 58 | Inquiry |
| Hebei Mojin Biotechnology Co., Ltd | +86 13288715578 +8613288715578 | China | 12460 | 58 | Inquiry |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | China | 21675 | 55 | Inquiry |
| Shanghai Time Chemicals CO., Ltd. | +86-021-57951555 +8617317452075 | China | 1807 | 55 | Inquiry |
| ATK CHEMICAL COMPANY LIMITED | +undefined-21-51877795 | China | 32760 | 60 | Inquiry |
| career henan chemical co | +86-0371-86658258 +8613203830695 | China | 29900 | 58 | Inquiry |
| Hebei Guanlang Biotechnology Co., Ltd. | +86-19930503282 | China | 8827 | 58 | Inquiry |
| Xiamen AmoyChem Co., Ltd | +86-592-6051114 +8618959220845 | China | 6387 | 58 | Inquiry |
| Chongqing Chemdad Co., Ltd | +86-023-6139-8061 +86-86-13650506873 | China | 39916 | 58 | Inquiry |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | China | 49391 | 58 | Inquiry |
10025-78-2(Trichlorosilane)Related Search:
1of4
chevron_right




