Silicon
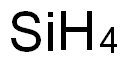
- CAS No.
- 7440-21-3
- Chemical Name:
- Silicon
- Synonyms
- silicium;SILICON METAL;Silica powder;silicon,powder;Silicon nanopowder;SILICON METAL POWDER;polycrystalline silicon;Silicon, powder, 99.9%;Silicon Monocrystalline;monocrystalline silicon
- CBNumber:
- CB4720557
- Molecular Formula:
- H4Si
- Molecular Weight:
- 32.12
- MOL File:
- 7440-21-3.mol
- MSDS File:
- SDS
- Modify Date:
- 2024/5/27 16:04:53
| Melting point | 1410 °C(lit.) |
|---|---|
| Boiling point | 2355 °C(lit.) |
| Density | 2.33 g/mL at 25 °C(lit.) |
| storage temp. | Flammables area |
| solubility | insoluble in H2O, acid solutions; soluble in alkaline solutions |
| form | powder |
| Specific Gravity | 2.42 |
| color | White |
| PH | 13.5 (H2O, 20°C) |
| Odor | Odorless |
| Water Solubility | INSOLUBLE |
| Sensitive | Air Sensitive |
| Crystal Structure | Cubic, Diamond Structure - Space Group Fd3m |
| Merck | 13,8565 |
| Dielectric constant | 2.4(Ambient) |
| Exposure limits |
ACGIH: TWA 2.5 mg/m3 NIOSH: IDLH 250 mg/m3; TWA 2.5 mg/m3 |
| Stability | Stable. Fine powder is highly flammable. Incompatible with oxidizing agents, bases, carbonates, alkali metals, lead and aluminium oxides, halogens, carbides, formic acid. |
| InChIKey | BLRPTPMANUNPDV-UHFFFAOYSA-N |
| CAS DataBase Reference | 7440-21-3(CAS DataBase Reference) |
| NIST Chemistry Reference | Silicon(7440-21-3) |
| EPA Substance Registry System | Silicon (7440-21-3) |
| Modulus of Elasticity | 112.4 GPa |
|---|---|
| Poissons Ratio | 0.28 |
| Shear Modulus | 43.9 GPa, Calculated |
| Hardness, Mohs | 7.0 |
| Knoop Microhardness | 11270, N/mm2 Microhardness |
| Bulk Modulus | 98.74 GPa |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS02 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H228 | |||||||||
| Precautionary statements | P210-P240-P241-P280-P370+P378 | |||||||||
| Hazard Codes | T,F | |||||||||
| Risk Statements | 11 | |||||||||
| Safety Statements | 26-36/37-45-7/9-33-16-36 | |||||||||
| RIDADR | UN 2922 8/PG 2 | |||||||||
| OEB | B | |||||||||
| OEL | TWA: 10 mg/m3 (total) | |||||||||
| WGK Germany | 2 | |||||||||
| RTECS | VW0400000 | |||||||||
| Autoignition Temperature | 780°C | |||||||||
| TSCA | Yes | |||||||||
| HS Code | 3822 00 00 | |||||||||
| HazardClass | 4.1 | |||||||||
| PackingGroup | III | |||||||||
| NFPA 704 |
|
Silicon price More Price(139)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 795585 | Silicon nanopowder, <100?nm (BET), <3% oxygen passivation | 7440-21-3 | 25G | ₹34561.8 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 795585 | Silicon nanopowder, <100?nm (BET), <3% oxygen passivation | 7440-21-3 | 25G | ₹34561.8 | 2022-06-14 | Buy |
| Sigma-Aldrich | 795585 | Silicon nanopowder, <100?nm (BET), <3% oxygen passivation | 7440-21-3 | 100G | ₹86267.35 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 795585 | Silicon nanopowder, <100?nm (BET), <3% oxygen passivation | 7440-21-3 | 100G | ₹86267.35 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 767492 | Silicon sputtering target, diam. × thickness 2.00?in. × 0.25?in., 99.999% trace metals basis | 7440-21-3 | 1EA | ₹24623.7 | 2022-06-14 | Buy |
Silicon Chemical Properties,Uses,Production
Chemical Properties
Silicon is a nonmetallic element which is known as silicon metal. Not occur freely in nature, but is found in silicon dioxide (silica) and in various silicates. It is a steel-gray crystalline solid or a black-brown amorphous material.
Physical properties
Silicon does not occur free in nature, but is found in most rocks, sand, and clay. Siliconis electropositive, so it acts like a metalloid or semiconductor. In some ways silicon resemblesmetals as well as nonmetals. In some special compounds called polymers, silicon will act inconjunction with oxygen. In these special cases it is acting like a nonmetal.
There are two allotropes of silicon. One is a powdery brown amorphous substance bestknown as sand (silicon dioxide). The other allotrope is crystalline with a metallic grayishluster best known as a semiconductor in the electronics industry. Individual crystals of siliconare grown through a method known as the Czochralski process. The crystallized siliconis enhanced by “doping” the crystals (adding some impurities) with other elements such asboron, gallium, germanium, phosphorus, or arsenic, making them particularly useful in themanufacture of solid-state microchips in electronic devices.
The melting point of silicon is 1,420°C, its boiling point is 3,265°C, and its density is2.33 g/cm3.
Isotopes
There are 21 isotopes of silicon, three of which are stable. The isotope Si-28makes up 92.23% of the element’s natural abundance in the Earth’s crust, Si-29 constitutes4.683% of all silicon found in nature, and the natural abundance of Si-30 ismerely 3.087% of the stable silicon isotopes found in the Earth’s crust.
Origin of Name
Silicon was named after the Latin word silex, which means “flint.”
Occurrence
Silicon, in the form of silicon dioxide (SiO2), is the most abundant compound in theEarth’s crust. As an element, silicon is second to oxygen in its concentration on Earth, yet it is only the seventh most abundant in the entire universe. Even so, silicon is used as the standard(Si = 1) to estimate the abundances of all other elements in the universe. For example, hydrogenequals 40,000 times the amount of silicon in the cosmos. Hydrogen is the most abundantof all elements in the universe, and carbon is just three and half times as abundant as siliconin the entire universe. On Earth silicon accounts for 28% of the crust, oxygen makes up 47%of the crust, and much of the rest of the crust is composed of aluminum.
It is believed that silicon is the product of the cosmic nuclear reaction in which alpha particleswere absorbed at a temperature of 109 Kelvin into the nuclei of carbon-12, oxygen-16,and neon-20. Pure elemental silicon is much too reactive to be found free in nature, but it doesform many compounds on Earth, mainly oxides as crystals (quartz, cristobalite, and tridymite)and amorphous minerals (agate, opal, and chalcedony). Elemental silicon is produced byreducing silica (SiO2) in a high-temperature electric furnace, using coke as the reducing agent.It is then refined. Silicon crystals used in electronic devices are “grown” by removing startercrystals from a batch of melted silicon.
Characteristics
The characteristics of silicon in some ways resemble those of the element germanium,which is located just below it in the carbon group.
Flint is the noncrystalline form of silicon and has been known to humans since prehistorictimes. When struck with a sharp blow, flint would flake off sharp-edged chips that were thenused as cutting tools and weapons.
In addition to silica (silicon dioxide SiO2), the crystal form of silicon is found in severalsemiprecious gemstones, including amethyst, opal, agate, and jasper, as well as quartz of varyingcolors. A characteristic of quartz is its piezoelectric effect. This effect occurs when thequartz crystal is compressed, producing a weak electrical charge. Just the opposite occurs whenelectric vibrations are fed to the crystal. These vibrations are then duplicated in the crystal.Quartz crystals are excellent timekeeping devices because of this particular characteristic.
Uses
In making silanes and silicones, the Si-C bond being about as strong as a C-C bond. In the manufacture of transistors, silicon diodes and similar semiconductors. For making alloys such as ferrosilicon, silicon bronze, silicon copper. As a reducing agent like aluminum in high tempereture reactions.
Definition
silicon: Symbol Si. A metalloid element belonging to group 14 (formerlyIVB) of the periodic table; a.n.14; r.a.m. 28.086; r.d. 2.33; m.p.1410°C; b.p. 2355°C. Silicon is thesecond most abundant element inthe earth’s crust (25.7% by weight) occurring in various forms of silicon(IV)oxide (e.g. quartz) and in silicateminerals. The element is extractedby reducing the oxide with carbon inan electric furnace and is used extensivelyfor its semiconductor properties.It has a diamond-like crystalstructure; an amorphous form alsoexists. Chemically, silicon is less reactivethan carbon. The element combineswith oxygen at red heat and isalso dissolved by molten alkali. Thereis a large number of organosiliconcompounds (e.g. siloxanes) althoughsilicon does not form the range ofsilicon–hydrogen compounds andderivatives that carbon does (seesilane). The element was identifiedby Antoine Lavoisier in 1787 andfirst isolated in 1823 by J?ns Berzelius.
Reactions
Si is a little soluble in water (Solubility: 0.005 g/100 g H2O (298 K)). It reacts strongly with F at room temperature, Cl at 430℃, Br at 500℃, O at 400℃, and N2 at 1000℃ to form compounds of SiF4 , SiCl4 , SiBr4 , SiO2 , SiO, and Si3N4 , respectively.
General Description
A dark brown powder. Insoluble in water and denser than water. Burns readily when exposed to heat or flames, and may be difficult to extinguish. Water may not be effective in extinguishing flames. Used to make computer microchips.
Air & Water Reactions
Highly flammable. Insoluble in water. A significant dust explosion hazard.
Reactivity Profile
Silicon is a reducing agent. Ignites in fluorine gas at ordinary temperatures [Mellor 2:11-13 1946-47]. Burns spontaneously in gaseous chlorine. A mixture of silicon, aluminum, and lead oxide explodes when heated [Mellor 7:657 1946-47]. When heated with an alkali carbonate, a vigorous reaction attended by incandescence occurs [Mellor 6:164 1946-47]. Reacts violently with silver fluoride [Mellor 3:389 1946-47]. Reacts with sodium-potassium alloy to form sodium silicide, which is spontaneously flammable in air [Mellor 2 Supp. 2:564 1961].
Hazard
The dust of silicon oxide (silicate) can burn or explode and is very harmful if inhaled.Continued exposure to silica dust causes silicosis, a form of pneumonia.
The hydrides of silicon (silicon plus hydrogen) are extremely volatile and spontaneouslyburst into flames in air at room temperatures. They must be kept in special vacuum chambers.
Over the past several decades, there has been some concern over the potential hazards andsafety of the cosmetic use of silicone body implants—breast implants, in particular. Severalmanufactures have been sued over the failure of the implants, and the federal government (FDA) withdrew its approval for their use. Congressional hearings with manufacturers in2005 produced new information that has reversed the FDA’s ban on their use—but only withcertain manufacturers of implants. The debate continues.
Health Hazard
Oxides from metallic fires are a severe health hazard. Inhalation or contact with substance or decomposition products may cause severe injury or death. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may cause pollution.
Fire Hazard
May react violently or explosively on contact with water. Some are transported in flammable liquids. May be ignited by friction, heat, sparks or flames. Some of these materials will burn with intense heat. Dusts or fumes may form explosive mixtures in air. Containers may explode when heated. May re-ignite after fire is extinguished.
Industrial uses
Silicon is, after oxygen, the second most abundant element in the earth s crust. It occurs in a range of minerals and sand (SiO2, quartz). Silicon can be extracted from silicates or sand by reducing SiO2 with coke at high temperatures at around 3000°C.Silicon is used in a wide variety of applications. In nature, silicon does not exist as the pure metal and most commonly occurs in silica (including sand) and silicates. Silicon dioxide, also known as silica, is a hard substance with a high melting temperature and clearly very different from carbon dioxide. Molten silica can be used to make glass, an extremely useful material, which is resistant to attack by most chemicals except fluorine, hydrofluoric acid and strong alkalis. Silicon atoms can also be found in the class of compounds called silicones. Pure silicon metal is used in semiconductors, the basis of all electronic devices, and is most well known for its application in solar panels and computer chips.
Safety Profile
A nuisance dust. Moderately toxic by ingestion. An eye irritant. Does not occur freely in nature, but is found as sdicon dioxide (sdtca) and as various shcates. Elemental Si is flammable when exposed to flame or by chemical reaction with oxidlzers. Violent reactions with alkali carbonates, oxidants, (A1 + PbO), Ca, Cs2C2, Cl2, CoF2, F2, IFs, MnF3, Rb2C2, FNO, AgF, NaK alloy. When heated it will react with water or steam to produce H2; can react with oxidizing materials. See also various silica entries, SILICATES, and POWDERED METALS.
Potential Exposure
Silicon may be used in the manufacture of silanes, silicon tetrachloride, ferrosilicon, silicones. It is used in purified elemental form in transistors and photovoltaic cells.
Shipping
UN1346 Silicon powder, amorphous requires, Hazard Class: 4.1; Labels: 4.1-Flammable solid.
Incompatibilities
Dust or powder may form explosive mixture with air. A strong reducing agent. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides, calcium, carbonates, chlorine, fluorine, oxidizers, cesium carbide; alkaline carbonates.
Silicon Preparation Products And Raw materials
Raw materials
1of2
chevron_rightPreparation Products
1of4
chevron_right| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Palmo Industrial Silicones Pvt. Ltd. | +91-652750050 +91-9825025930 | Vadodara, India | 3 | 58 | Inquiry |
| The Metal Powder Company Limited | +91-4549280595 +91-4549280599 | Tamil Nadu, India | 16 | 58 | Inquiry |
| Silicon Products (P) Associates | +91-8591240288 +91-9324400900 | Maharashtra, India | 2 | 58 | Inquiry |
| ALPHA CHEMIKA | +91-22-22061123 +91-22-66382501 | Mumbai, India | 1681 | 43 | Inquiry |
| Scientific OEM | +91-22- 2343 7546 / 2341 3094 | New Delhi, India | 1996 | 38 | Inquiry |
| Sisco Research Laboratories Pvt. Ltd. | +91-22-4268 5800 | Mumbai, India | 4317 | 58 | Inquiry |
| Triveni chemicals | 08048762458 | New Delhi, India | 6093 | 58 | Inquiry |
| LOBA CHEMIE PVT.LTD. | 91-22-6663 6699 | Mumbai, India | 3077 | 58 | Inquiry |
| Central Drug House(P) Ltd. | 91-11-49404040 | New Delhi, India | 6160 | 58 | Inquiry |
| Aritech Chemazone Private Limited | +91-9034345475 | Punjab, India | 684 | 58 | Inquiry |
Related articles
- Fun Facts About Silicon
- Numerous silicate minerals have direct commercial applications, for example, clays, silica sand, and most kinds of building st....
- May 27,2024
- Silicon--diamond cubic crystal structure
- Silicon (Si) is a nonmetallic chemical element in the carbon family. Silicon makes up 27.7 percent of Earth's crust, the secon....
- May 8,2024
- What is the ionic charge of silicon?
- Silicon typically forms ions with a charge of +4. This means it can lose four electrons to achieve a stable electron configura....
- Feb 7,2024
7440-21-3(Silicon)Related Search:
1of4
chevron_right




