Copper(I) Cyanide
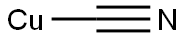
- CAS No.
- 544-92-3
- Chemical Name:
- Copper(I) Cyanide
- Synonyms
- COPPER CYANIDE;CUPROUS CYANIDE;Cu(CN);CopperCyanideTech.;Copper cyanide (Cu(CN));CUPRICIN;ai3-28745;CUCURBITACINA;Cyanocopper(I);Coprous cyanide
- CBNumber:
- CB0193966
- Molecular Formula:
- CCuN
- Molecular Weight:
- 89.56
- MOL File:
- 544-92-3.mol
- MSDS File:
- SDS
- Modify Date:
- 2024/3/14 15:18:25
| Melting point | 474 °C(lit.) |
|---|---|
| Boiling point | decomposes [STR93] |
| Density | 2.92 g/mL at 25 °C(lit.) |
| vapor pressure | 0Pa at 25℃ |
| storage temp. | Poison room |
| solubility | insoluble in H2O, ethanol; soluble in KCN solution |
| form | Powder |
| Specific Gravity | 2.92 |
| color | White-beige to greenish |
| Water Solubility | Practically insoluble in water and alcohol. Soluble in ammonium hydroxide, aqueous ammonia, pyridine and N-methylpyrrolidone. |
| Sensitive | air sensitive |
| Merck | 14,2661 |
| BRN | 3587244 |
| Solubility Product Constant (Ksp) | pKsp: 19.46 |
| Exposure limits | TLV-TWA 1 mg Cu/m3 (ACGIH). |
| Stability | Stable. Incompatible with acids, bases, magnesium. Reacts violently with oxidizing agents, nitrates. Reaction with acid releases highly toxic gas (HCN). |
| LogP | -1.49 at 25℃ |
| CAS DataBase Reference | 544-92-3(CAS DataBase Reference) |
| EPA Substance Registry System | Copper(I) cyanide (544-92-3) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS06,GHS09 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H300+H310+H330-H410 | |||||||||
| Precautionary statements | P260-P262-P273-P280-P302+P352+P310-P304+P340+P310 | |||||||||
| Hazard Codes | T+,N,T | |||||||||
| Risk Statements | 26/27/28-32-50/53 | |||||||||
| Safety Statements | 7-28-29-45-60-61-28A | |||||||||
| RIDADR | UN 1587 6.1/PG 2 | |||||||||
| WGK Germany | 2 | |||||||||
| RTECS | GL7150000 | |||||||||
| F | 10-23 | |||||||||
| TSCA | Yes | |||||||||
| HS Code | 2837 19 00 | |||||||||
| HazardClass | 6.1 | |||||||||
| PackingGroup | II | |||||||||
| NFPA 704 |
|
Copper(I) Cyanide price More Price(8)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | 216305 | Copper(I) cyanide 99% | 544-92-3 | 100G | ₹3334.1 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 216305 | Copper(I) cyanide 99% | 544-92-3 | 5G | ₹4925.38 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 216305 | Copper(I) cyanide 99% | 544-92-3 | 500G | ₹5196 | 2022-06-14 | Buy |
| ALFA India | ALF-012135-F3 | Copper(I) cyanide | 544-92-3 | 2x2.5kg | ₹38707 | 2022-05-26 | Buy |
| ALFA India | ALF-012135-A1 | Copper(I) cyanide | 544-92-3 | 1kg | ₹9557 | 2022-05-26 | Buy |
Copper(I) Cyanide Chemical Properties,Uses,Production
Description
off-white to green powder. Insoluble in water,soluble in HCI, Nl40H, and potassium cyanide. Used in Sandmeyer's reaction to synthesize aryl cyanides. Toxic by skin absorption, through open wounds, by ingestion, and by inhalation of hydrogen cyanide that arises from slight decomposition. Produces toxic oxides of nitrogen in fires.
Chemical Properties
Cuprous cyanide is a white crystalline substance.
Physical properties
Cream-colored powder or green orthorhombic or red monoclinic crystals; density 2.90 g/cm3; melts at 474°C; decomposes at higher temperatures; practically insoluble in water, ethanol, and cold dilute acids; dissolves in ammonium hydroxide and potassium cyanide solutions.
Uses
Copper(I) cyanide is commonly used as a polymerization catalyst. The compound is useful in organic synthesis, as a catalyst, reagent in the preparation of nitriles(sandmeyer reaction). It is also used in electroplating copper and iron, silver plating, brass plating, and copper-tin alloy plating. It is used to make phthalocyanine dyes and pigments.
Reactivity Profile
Copper(I) Cyanide is decomposed by acids to give off hydrogen cyanide, a flammable poisonous gas. Tends to explosive instability. Capable of violent oxidation under certain condition: fusion with metal chlorates, perchlorates, nitrates or nitrites can cause explosions [Bretherick, 1979 p. 101]. Reacts with incandescence with magnesium [Mellor, 1940, Vol. 4, 271].
Hazard
Poison.
Health Hazard
Cuprous cyanide is a highly toxic substance. The toxic routes are inhalation of dust, ingestion, and skin contact. Toxicology and LD50 values for this compound are not reported. Because it is slightly soluble in water, its dissociation to cuprous and cyanide ions in the body may not be significant. The role of cyanide ion in the toxicity of cuprous cyanide is not established. The inhalation hazard, however, is attributable to copper. It is a skin irritant.
Fire Hazard
Special Hazards of Combustion Products: Toxic hydrogen cyanide gas may form in fires.
Safety Profile
A poison. Reacts violently with magnesium. When heated to decomposition it emits very toxic CNand NOx. See also CYANIDE and COPPER COMPOUNDS.
Potential Exposure
Copper cyanide is used in electroplating copper on iron; and as an insecticide and a catalyst.
Shipping
UN1587 Copper cyanide, Hazard Class: 6.1; Labels: 6.1-Poisonous materials
Purification Methods
Wash the cyanide thoroughly with boiling H2O, then with EtOH. Dry it at 100o to a fine soft powder. It dissolves in excess alkali cyanide solutions to form the very soluble complex ion Cu(CN)43-. [Bassett & Corbett J Chem Soc 125 1660 1924, Barber J Chem Soc 79 1943.]
Incompatibilities
Contact with heat, strong acids (HCl, H2SO4, HNO3) forms deadly hydrogen cyanide gas. May release hydrogen cyanide on contact with moisture. Incompatible with strong oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides, acetylene gas, and chemically active metals, such as potassium, sodium, magnesium, and zinc.
Waste Disposal
Copper-containing soluble wastes can be concentrated through the use of ion exchange, reverse osmosis, or evaporators to the point where copper can be electrolytically removed and sent to a reclaiming firm. If recovery is not feasible, the copper can be precipitated through the use of caustics and the sludge deposited in a chemical waste landfill. Copper-containing wastes can be concentrated to the point where copper can be electrolytically removed and reclaimed. If recovery is not feasible, the copper can be precipitated by alkali; the cyanide destroyed by alkaline oxidation yielding a sludge which can be sent to a chemical waste landfill. In accordance with 40CFR165, follow recommendations for the disposal of pesticides and pesticide containers. Must be disposed properly by following package label directions or by contacting your local or federal environmental control agency, or by contacting your regional EPA office.
Copper(I) Cyanide Preparation Products And Raw materials
Raw materials
1of3
chevron_rightPreparation Products
1of8
chevron_right| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| ANJI BIOSCIENCES | +91-9000100077 +91-9000100077 | Hyderabad, India | 430 | 58 | Inquiry |
| JAGGO OVERSEAS | +91-9999536088 +91-9999536089 | New Delhi, India | 6 | 58 | Inquiry |
| JSK Chemicals | +919879767970 | Gujarat, India | 3756 | 58 | Inquiry |
| DL INTRACHEM | +91-9375014678 +91-9099014678 | Gujarat, India | 55 | 58 | Inquiry |
| Eureka Chemicals | +91-2222074176 +91-2222074206 | Maharashtra, India | 11 | 58 | Inquiry |
| Samuh Laxmi Chemicals (Bom) P Ltd | +91-9004585338 +91-8369298209 | Maharashtra, India | 25 | 58 | Inquiry |
| S D Fine Chem Limited | +91-9323715856 +91-9323715856 | Maharashtra, India | 368 | 58 | Inquiry |
| Clearsynth Labs | 91-22-45045900 | Maharashtra, India | 3889 | 58 | Inquiry |
| Kemona Impex | 08068441141Ext 577 | Mumbai, India | 221 | 58 | Inquiry |
| ASM Organics | 91-9866122393 | Andhra Pradesh, India | 1188 | 58 | Inquiry |
| Supplier | Advantage |
|---|---|
| ANJI BIOSCIENCES | 58 |
| JAGGO OVERSEAS | 58 |
| JSK Chemicals | 58 |
| DL INTRACHEM | 58 |
| Eureka Chemicals | 58 |
| Samuh Laxmi Chemicals (Bom) P Ltd | 58 |
| S D Fine Chem Limited | 58 |
| Clearsynth Labs | 58 |
| Kemona Impex | 58 |
| ASM Organics | 58 |
544-92-3(Copper(I) Cyanide)Related Search:
1of4
chevron_right




