Barium hydroxide
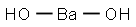
- CAS No.
- 17194-00-2
- Chemical Name:
- Barium hydroxide
- Synonyms
- Ba(OH)2;Anhydrous Barium hydroxide;Aetzbaryt;Chebi:32592;BARIUM HYDRATE;baryta hydrate;Bariumhydroxid;BARIUM HYDROXIDE;bariumdihydroxide;Barium dihydoxide
- CBNumber:
- CB5118700
- Molecular Formula:
- BaH2O2
- Molecular Weight:
- 171.34
- MOL File:
- 17194-00-2.mol
- MSDS File:
- SDS
- Modify Date:
- 2024/3/21 15:24:59
| Melting point | >300 °C(lit.) |
|---|---|
| Boiling point | decomposes at 1032 (calculated) [KNA91] |
| Density | 2.2 g/mL at 25 °C(lit.) |
| solubility | Aqueous Acid (Slightly), Water (Slightly) |
| form | Solid |
| color | Clear |
| PH | 11.27(1 mM solution);12.22(10 mM solution);13.08(100 mM solution) |
| Odor | no odor |
| Water Solubility | Soluble in water. |
| Sensitive | Air Sensitive & Hygroscopic |
| Merck | 14,977 |
| Exposure limits |
ACGIH: TWA 0.5 mg/m3 NIOSH: IDLH 50 mg/m3; TWA 0.5 mg/m3 |
| Stability | Stable. Incompatible with acids, carbon dioxide, moisture. |
| InChIKey | RQPZNWPYLFFXCP-UHFFFAOYSA-L |
| CAS DataBase Reference | 17194-00-2(CAS DataBase Reference) |
| NIST Chemistry Reference | Barium dihydroxide(17194-00-2) |
| EPA Substance Registry System | Barium hydroxide (Ba(OH)2) (17194-00-2) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS05,GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H302+H332-H314 | |||||||||
| Precautionary statements | P260-P280-P301+P312-P303+P361+P353-P304+P340+P310-P305+P351+P338 | |||||||||
| Hazard Codes | C,Xi,Xn | |||||||||
| Risk Statements | 36/37/38-34-20/22-36/38-41-35-22 | |||||||||
| Safety Statements | 26-36/37/39-45-36/37 | |||||||||
| RIDADR | UN 3262 8/PG 2 | |||||||||
| WGK Germany | 1 | |||||||||
| RTECS | CQ9200000 | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 8 | |||||||||
| PackingGroup | III | |||||||||
| HS Code | 2805191000 | |||||||||
| Toxicity | LD50 ipr-mus: 255 mg/kg GTPZAB 28(6),45,84 | |||||||||
| NFPA 704 |
|
Barium hydroxide price More Price(8)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | 433373 | Barium hydroxide technical grade, ~95% | 17194-00-2 | 250G | ₹3464 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | B4059 | Barium hydroxide solution 0.3?N | 17194-00-2 | 500ML | ₹10695.1 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 433373 | Barium hydroxide technical grade, ~95% | 17194-00-2 | 1KG | ₹8162.05 | 2022-06-14 | Buy |
| ALFA India | ALF-012195-A1 | Barium hydroxide, anhydrous, 94-98% | 17194-00-2 | 1kg | ₹8907 | 2022-05-26 | Buy |
| ALFA India | ALF-012195-30 | Barium hydroxide, anhydrous, 94-98% | 17194-00-2 | 250g | ₹4798 | 2022-05-26 | Buy |
Barium hydroxide Chemical Properties,Uses,Production
Description
Barium Hydroxide occurs as an anhydrate whose prismatic, colorless
crystals are very deliquescent. Barium hydroxide can
be prepared by dissolving barium oxide (BaO) in water:
BaO+ 9H2O→Ba(OH)2·8H2O
It crystallizes as the octahydrate, which converts to
the monohydrate upon heating in air. Barium oxide
has been found to react with water vapor at temperatures
from 1443 to 1593 K to form gaseous Ba(OH)2
according to the reaction:
BaO(solid)+H2O(gas)→Ba(OH)2(gas)
A better method of preparation involves addition of
NaOH to the nitrate. Since barium hydroxide is fairly
soluble, it is necessary to evaporate the solution to crystallize Ba(OH)2·8H2O. Ethanol is added to aid in this crystallization. The crystals are washed in cold ethanol to remove sodium ions and then dried.
Chemical Properties
Barium hydroxide,Ba(OH)2·8H20, is a white crystalline powder, colorless solid composed of monoclinic crystals. It has a melting point of 78°C and is soluble in water and insoluble in acetone. It is used in the fusion of silicates and fat saponification.
Physical properties
Monohydrate, Ba(OH)2?H2O is a white powder; density 3.743 g/cm3; slightly soluble in water; soluble in dilute mineral acids. Octahydrate, Ba(OH)2?8H2O is a colorless monoclinic crystal; density 2.18 g/cm3 at 16°C; refractive index 1.50; melts at 78°C; vapor pressure 227 torr; loses seven molecules of water of crystallization when its solution is boiled in the absence of atmospheric CO2 forming solid monohydrate; further heating produces anhydrous Ba(OH)2 melting at 407°C; readily dissolves in water (3.76 g/100 g at 20°C and 11.7 g/100 g at 50°C); aqueous solution highly alkaline; also soluble in methanol; slightly soluble in ethanol; insoluble in acetone.
Uses
Barium hydroxide is used in analytical chemistry to titrate weak acids, particularly organic acids. It forms clear aqueous solutions that are free from carbonate, unlike those of the alkali hydroxides, since barium carbonate is insoluble in water. This allows the use of indicators such as phenolphthalein or thymophthalein (with alkaline color changes) without the risk of titration errors due to the presence of weakly basic CO32- ions.Barium hydroxide is used in organic synthesis as a strong base, for example for the hydrolysis of esters and nitriles.It has been used to hydrolyze one of the two equivalent ester groups in dimethyl endecanedioate. It is also used in the preparation of cyclopentanone, diacetone alcohol and d-Gluonic g-lactone.
Barium hydroxide is used as an additive in thermoplastics (such as phenolic resins), rayon and PVC stabilizers to improve plastic properties. This material is used as a general-purpose additive for lubricants and greases. Other industrial applications for barium hydroxide include sugar fabrication, manufacturing soaps, fat saponification, fusing of silicates and chemical synthesis of other barium compounds and organic compounds.
Barium hydroxide is offered for sale both as the octahydrate and the monohydrate. It is available in large quantities commercially.
Preparation
Barium hydroxide is made by dissolving barium oxide in hot water. The octahydrate, Ba(OH)2?8H2O, crystallizes upon cooling. It also is prepared by precipitation with caustic soda from an aqueous solution of barium sulfide:
BaS + 2NaOH → Ba(OH)2 + Na2S.
Hazard
An acute poison; toxic symptoms are similar to other soluble salts of barium (see Barium).
Safety Profile
A poison by ingestion andintraperitoneal routes. Incompatible with chlorinated rubber.
Barium hydroxide Preparation Products And Raw materials
Raw materials
Preparation Products
1of8
chevron_right| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| ALLIPO CHEMICALS | +91-8238998187 +91-8238998187 | Gujarat, India | 32 | 58 | Inquiry |
| Kronox Lab Sciences Pvt Ltd | +91-9313231074 +91-9313231074 | Gujarat, India | 240 | 58 | Inquiry |
| Divjyot Chemicals Private Limited | +91-8048603207 +91-9849191943 | Telangana, India | 11 | 58 | Inquiry |
| Shiv Organo Synthesis | +91-9825915701 +91-9825740398 | Gujarat, India | 7 | 58 | Inquiry |
| Nithyasri Chemicals (APURVA CHEMICALS) | +91-2512692130 +91-9323652199 | Maharashtra, India | 75 | 58 | Inquiry |
| Sanchem Sophin Pvt. Ltd., (Earlier M/s. Sandeep Enterprises) | 91-40-27892181 | Telangana, India | 58 | 58 | Inquiry |
| Kronox Lab Sciences Pvt., Ltd. | 91-2662-244077 | Gujarat, India | 53 | 58 | Inquiry |
| Perfect Chemical | 08048372764Ext 379 | Mumbai, India | 171 | 58 | Inquiry |
| Ganesh Trading Company | 08046054050 | Mumbai, India | 35 | 58 | Inquiry |
| H. M. Dye Chem. | 91-22-66108167 | Maharashtra, India | 15 | 58 | Inquiry |
Related Qustion
- Q:Is Ba(OH)2 a strong base?
- A:As the metal it is made of (barium) sits at the bottom of Group II, its hydroxide is considerably strong and comparable to Gro....
- Mar 21,2024
17194-00-2(Barium hydroxide )Related Search:
1of4
chevron_right




