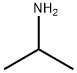
Isopropylamine
- CAS No.
- 75-31-0
- Chemical Name:
- Isopropylamine
- Synonyms
- MIPA;MONOISOPROPYLAMINE;2-Propanamine;2-Propylamine;Isopropylamine Anhydrous;sopropylamine;MIPA-70;nsc62775;iso-C3H7NH2;i-Propylamin
- CBNumber:
- CB8854223
- Molecular Formula:
- C3H9N
- Molecular Weight:
- 59.11
- MOL File:
- 75-31-0.mol
- MSDS File:
- SDS
- Modify Date:
- 2024/5/23 18:06:45
| Melting point | -101 °C |
|---|---|
| Boiling point | 32-35 °C 33-34 °C (lit.) |
| Density | 0.688 g/mL at 20 °C (lit.) |
| vapor density | 2.04 (vs air) |
| vapor pressure | 9.2 psi ( 20 °C) |
| FEMA | 4238 | ISOPROPYLAMINE |
| refractive index |
n |
| Flash point | −26 °F |
| storage temp. | 2-8°C |
| solubility | 1000g/l |
| form | Crystalline Powder, Needles or Crystals |
| pka | 10.63(at 25℃) |
| color | APHA: ≤50 |
| Odor | Strong ammoniacal; pungent, irritating, typical amine. |
| PH | 13 (700g/l, H2O, 20℃) |
| explosive limit | 2-10.4%(V) |
| Odor Threshold | 0.025ppm |
| Odor Type | fishy |
| Water Solubility | soluble |
| Sensitive | Air Sensitive |
| JECFA Number | 1581 |
| Merck | 14,5209 |
| BRN | 605259 |
| Exposure limits | TLV-TWA 5 ppm (~12 mg/m3) (ACGIH, MSHA, and OSHA); TLV-STEL 10 ppm(~24 mg/m3) (ACGIH); IDLH 4000 ppm (NIOSH). |
| Dielectric constant | 5.5(20℃) |
| Stability | Stable. Extremely flammable - note low boiling point and low flash point. Readily forms explosive mixtures with air. Incompatible with strong oxidizing agents, acids, acid chlorides, acid anhydrides, perchloryl fluoride. |
| LogP | 0.21 |
| CAS DataBase Reference | 75-31-0(CAS DataBase Reference) |
| NIST Chemistry Reference | 2-Propanamine(75-31-0) |
| EPA Substance Registry System | Isopropylamine (75-31-0) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS02,GHS05,GHS06 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H224-H301+H311+H331-H314-H335 | |||||||||
| Precautionary statements | P210-P233-P280-P303+P361+P353-P304+P340+P310-P305+P351+P338-P403+P233 | |||||||||
| Hazard Codes | F+,Xi,T | |||||||||
| Risk Statements | 12-36/37/38-37-35-25-20/21 | |||||||||
| Safety Statements | 16-26-29-45-36/37/39 | |||||||||
| RIDADR | UN 1221 3/PG 1 | |||||||||
| WGK Germany | 1 | |||||||||
| RTECS | NT8400000 | |||||||||
| F | 34 | |||||||||
| Autoignition Temperature | 755 °F | |||||||||
| TSCA | Yes | |||||||||
| HS Code | 2921 19 99 | |||||||||
| HazardClass | 3 | |||||||||
| PackingGroup | I | |||||||||
| Toxicity | LD50 orally in rats: 820 mg/kg (Smyth) | |||||||||
| IDLA | 750 ppm | |||||||||
| NFPA 704 |
|
Isopropylamine price More Price(17)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | 8.07476 | Isopropylamine for synthesis | 75-31-0 | 100ML | ₹4480 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 8.07476 | Isopropylamine for synthesis | 75-31-0 | 1L | ₹9019.99 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 8.07476 | Isopropylamine for synthesis | 75-31-0 | 2.5L | ₹10230.01 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 471291 | Isopropylamine ≥99.5% | 75-31-0 | 25ML | ₹2987.7 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 59330 | Isopropylamine ≥97.0% (GC) | 75-31-0 | 1L | ₹3431.53 | 2022-06-14 | Buy |
Isopropylamine Chemical Properties,Uses,Production
Description
Isopropylamine (propan-2-amine, IUPAC) is a colorless, volatile liquid. It is highly flammable, with a flammable range of 2%–10.4% in air. Boiling point is 93°F (33°C), flash point is ?15°F (?26°C), and ignition temperature is 756°F (402°C).
It is miscible with water, with a specific gravity of 0.69, which is lighter than water. Vapor density is 2.04, which is heavier than air. In addition to flammability, isopropylamine is a strong irritant to tissue and has a TLV of 5 ppm in air. The four-digit UN identification number is 1221. The NFPA 704 designation for isopropylamine is health 3, flammability 4, and reactivity 0. Primary uses for isopropylamine are pharmaceuticals, dyes, insecticides, and as a dehairing agent.
Chemical Properties
Isopropylamine is a colorless, flammable liquid. Isopropylamine is miscible with water, alcohol, and ether.The odor threshold reportedly ranges from 0.21 to 0.70 ppm; the pungent, ammoniacal odor becomes irritating at 24mg/m3 (110).
Physical properties
Colorless liquid with a penetrating, ammonia-like odor. Experimentally determined detection and recognition odor threshold concentrations were 500 μg/m3 (210 ppbv) and 1.7 mg/m3 (700 ppbv), respectively (Hellman and Small, 1974). An odor threshold concentration of 25 ppbv was reported by Nagata and Takeuchi (1990).
Occurrence
Not reported found in natu
Uses
Isopropylamine is used as a dehairing agentand as an intermediate in the preparation ofmany organics.
Uses
Isopropylamine is an organic compound is a widely used for the synthesis of pharmaceutical and agricultural goods such as glyphosphate herbicides and as an additive for petroleum industry. such as bentazon (BASF), Roundup (Monsanto), imazapyr (ACC) and the triazines ametryne (Novartis), atrazine (Novartis), desmetryn (Novartis), prometryn (Novartis), pramitol (Novartis), dipropetryn (Novartis), and propazine. Other applications encompass the nematicide fenamiphos (Bayer), the fungicide iprodione (Rho?ne-Poulenc), insecticides, pharmaceuticals, and surfactants.
Production Methods
Isopropylamine can be produced from the corresponding alcohol by reacting with ammonia in the presence of a dehydrating catalyst, or from the chloride by reacting with ammonia under pressure. It is also reported that this amine can be produced from acetone and ammonia or from the acetone oxime (HSDB 1989).
Definition
ChEBI: A member of the class of alkylamines that is propane carrying an amino group at position 2.
General Description
A clear colorless liquid with an ammonia-like odor. Flash point -35°F. Boiling point 90°F. Less dense than water Vapors heavier than air. Produces toxic oxides of nitrogen during combustion. Used as a solvent and to make other chemicals.
Air & Water Reactions
Highly flammable. Water soluble.
Reactivity Profile
Isopropylamine is a colorless, alkaline liquid, very volatile, moderately toxic, highly flammable. Dangerous fire hazard when exposed to heat, flame, sparks, or strong oxidizers. When heated to decomposition Isopropylamine emits toxic fumes of oxides of nitrogen [M. K.]. A mixture of Isopropylamine and perchloryl fluoride resulted in an uncontrolled oxidation and/or explosion, [J. Org. Chem., 1980, 45, 4036]. The reaction of 1-chloro-2,3-epoxypropane and the amine and most probably other nitrogen bases, yields a violent exotherm, [Chem. & Ind., 1971, 994].
Hazard
Highly flammable, dangerous fire risk. Strong irritant to tissue.
Health Hazard
Isopropylamine is a strong irritant to theeyes, skin, and respiratory system. A shortexposure to 10–20 ppm can cause irritationof the nose and throat in humans (Procturand Hughes 1978). Prolonged exposure tohigh concentrations may lead to pulmonaryedema. Skin contact can cause dermatitisand skin burns. Exposure to 8000 ppm for4 hours was lethal to rats.
LD50 value, oral (mice): 2200 mg/kg.
Chemical Reactivity
Reactivity with Water No reaction; Reactivity with Common Materials: No reactions; Stability During Transport: Stable; Neutralizing Agents for Acids and Caustics: Not pertinent; Polymerization: Not pertinent; Inhibitor of Polymerization: Not pertinent.
Industrial uses
Isopropylamine can be used as a dehairing agent and as a solvent. It also finds use as an intermediate in the production of insecticides, herbicides and bactericides and in the production of pharmaceuticals, dyes and rubber accelerators (HSDB 1989).
Environmental Fate
Photolytic. Low et al. (1991) reported that the photooxidation of aqueous primary amine
solutions by UV light in the presence of titanium dioxide resulted in the formation of ammonium
and nitrate ions.
Chemical/Physical. Releases toxic nitrogen oxides when heated to decomposition (Sax and
Lewis, 1987). Forms water-soluble salts with acids.
Metabolism
One would expect isopropylamine to be readily absorbed from the gut and respiratory tract. Shorter chain aliphatic amines such as isopropylamine also are efficiently absorbed through the skin (Beard and Noe 1981). When administered intravenously, isopropylamine distributed rapidly into tissue compartments with tissue/plasma ratios ranging from 1.8 in the atrium to 16.7 in the renal medulla (Privitera et al 1982). During the elimination phase, a half-life of 146 min was observed in plasma. There do not appear to be any definitive metabolic studies with this compound, however through a comparison with other substrates, one might expect oxidation to acetone and ammonia through the action of monoamine oxidase (Tipton 1980).
Isopropylamine Preparation Products And Raw materials
Raw materials
1of2
chevron_rightPreparation Products
1of8
chevron_right| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| JSK Chemicals | +919879767970 | Gujarat, India | 3756 | 58 | Inquiry |
| Scientific OEM | +91-22- 2343 7546 / 2341 3094 | New Delhi, India | 1996 | 38 | Inquiry |
| ALPHA CHEMIKA | +91-22-22061123 +91-22-66382501 | Mumbai, India | 1681 | 43 | Inquiry |
| Dr Prem's Molecules Pvt. Ltd. | +91 9825310038 | New Delhi, India | 547 | 50 | Inquiry |
| TCI Chemicals (India) Pvt. Ltd. | 1800 425 7889 | New Delhi, India | 6778 | 58 | Inquiry |
| Otto Chemie Pvt. Ltd. | +91 9820041841 | Mumbai, India | 5873 | 58 | Inquiry |
| Alfa Aesar | 1 800 209 7001 | Maharashtra, India | 6913 | 58 | Inquiry |
| Sisco Research Laboratories Pvt. Ltd. | +91-22-4268 5800 | Mumbai, India | 4317 | 58 | Inquiry |
| SynZeal Research Pvt Ltd | +1 226-802-2078 | Gujarat, India | 6522 | 58 | Inquiry |
| Pharmaffiliates Analytics and Synthetics P. Ltd | +91-172-5066494 | Haryana, India | 6773 | 58 | Inquiry |
75-31-0(Isopropylamine)Related Search:
1of4
chevron_right




