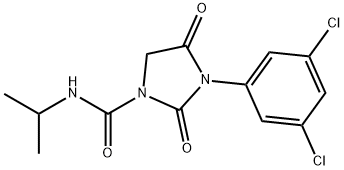
Iprodione
- CAS No.
- 36734-19-7
- Chemical Name:
- Iprodione
- Synonyms
- IPRODION;3-(3,5-dichlorophenyl)-1-iso-propylcarbamoylhydantion;3-(3,5-Dichlorophenyl)-N-(1-methylethyl)-2,4-dioxo-1-imidazolidinecarboxamide;KIDAN;ROVER;anfor;rovrol;ROVRAL;330.16;fa2071
- CBNumber:
- CB7131064
- Molecular Formula:
- C13H13Cl2N3O3
- Molecular Weight:
- 330.17
- MOL File:
- 36734-19-7.mol
- MSDS File:
- SDS
- Modify Date:
- 2025/1/27 9:38:02
| Melting point | 130-134 °C(lit.) |
|---|---|
| Density | 1.6223 (rough estimate) |
| vapor pressure | 5 x 10-7 Pa (25 °C) |
| refractive index | 1.6140 (estimate) |
| Flash point | 2 °C |
| storage temp. | Sealed in dry,Room Temperature |
| solubility | DMSO: 100 mg/mL (302.87 mM) |
| pka | 9.19±0.20(Predicted) |
| form | Solid |
| color | White to off-white |
| Water Solubility | 0.0013 g/100 mL |
| Merck | 13,5096 |
| BRN | 895003 |
| LogP | 3.000 |
| CAS DataBase Reference | 36734-19-7(CAS DataBase Reference) |
| NIST Chemistry Reference | Iprodione(36734-19-7) |
| EPA Substance Registry System | Iprodione (36734-19-7) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS08,GHS09 |
|---|---|
| Signal word | Warning |
| Hazard statements | H351-H410 |
| Precautionary statements | P202-P273-P280-P308+P313-P391-P405 |
| Hazard Codes | Xn,N,F |
| Risk Statements | 40-50/53-36-20/21/22-11 |
| Safety Statements | 36/37-60-61-36-26-16 |
| RIDADR | UN 3077 9/PG 3 |
| WGK Germany | 3 |
| RTECS | NI8870000 |
| HS Code | 29332900 |
| Hazardous Substances Data | 36734-19-7(Hazardous Substances Data) |
| Toxicity | LD50 in mice, rats (g/kg): 4, 3.5 orally (Lacroix, 1980) |
Iprodione price More Price(2)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | 559792 | [3-(3,5-Dichlorophenyl)-2,4-dioxoimidazolidinyl]-N-(methylethyl)carboxamide 97% | 36734-19-7 | 25G | ₹31479.1 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 36132 | Iprodione PESTANAL?, analytical standard | 36734-19-7 | 100MG | ₹6051.18 | 2022-06-14 | Buy |
Iprodione Chemical Properties,Uses,Production
Uses
Iprodione is a non-systemic fungicide that exhibits both protectant and eradicant activities against the spores and mycelium of a number of parasitic fungi. It is effective against Alternaria, Botrytis, Corticium, Fusarium, Helminthosporium, Monilinia, Phoma, Pleiochaeta, Rhizoctonia and Sclerotinia, etc. in nut crops, fruit trees (stone and pome fruit), vines, berries, vegetables, cereals, oilseed rape, cotton and ornamentals. Iprodione also controls various summer and winter turf diseases.
Definition
ChEBI: An imidazolidine-2,4-dione in which the nitrogen at position 1 is substituted by an N-(isopropyl)carboxamide group while that at position 3 is substituted by a 3,5-dichlorophenyl group. A contact fungicide, it blocks the growth of the fu gal mycelium and inhibits the germination of fungal spores. It is used on fruit and vegetable crops affected by various fungal diseases. It is also used as a nematicide.
Production Methods
The synthesis uses 3,5-dichloroaniline which is coupled with glycine and phosgene. The urea substructure is formed by reaction with isopropylamine and phosgene. Many fungicide mixtures with Iprodione are on the market.
Safety Profile
Moderately toxic by ingestion. When heated to decomposition it emits very toxic fumes of NOx and Cl-.
Environmental Fate
Soil. Readily degrades in soil (half-life 20 to 160 days) releasing carbon dioxide 3,5dichloroaniline (Walker, 1987) and (Hartley and Kidd, 1987; Worthing and Hance, 1991). The rate of degradation increases with repeated applications of this fungicide. In a clay loam, the half-life was 1 week. After the second and third applications, the half-lives were 5 and 2 days, respectively (Walker et al., 1986).
Plant. Translocation and uptake by potato plants were reported (Cayley and Hide, 1980). Iprodione is rapidly metabolized in plants to 3,5-dichloroaniline (Cayley and Hide, 1980; Hartley and Kidd, 1987).
Chemical/Physical. In an aqueous solution at pH 8.7, iprodione hydrolyzed to N-(3,5dichloroanilinocarbonyl)-N-(isopropylaminocarbonyl)glycine (Belafdal et al., 1986). At pH 8.7, complete hydrolysis occurred after 14 hours (Cayley and Hide, 1980).
Gomez et al. (1982) studied the pyrolysis of iprodione in an helium atmosphere at 400–1,000°C. Decomposition began at 300°C producing isopropyl isocyanate and 3-(3,5dichlorophenyl)hydantoin. Above 600°C, the hydantoin ring began to decompose forming the following products: 3-chloroaniline, 3,5-dichloroaniline, chlorinated benzenes and benzonitrile. From 800 to 1,000°C, the hydantoin ring was completed destroyed whichled to the formation of aryl isocyanates, anilines and the corresponding diarylureas, namely 3-(3,5-dichlorophenyl)urea and 1-(3-chlorophenyl)-3-(3,5-dichlorophenyl)urea (Gomez et al., 1982).
Metabolic pathway
The opening and rearrangement of the dioxoimidazolidine ring is the initial and major degradation/metabolic reaction for iprodione. Iprodione degrades in soil via cleavage of the dioxoimidazolidine-carboxamide linkage, followed by ring opening to yield 3,5-dichloroaniline and CO2. In plants and animals, primary degradation reactions include N-dealkylation of the isopropyl moiety, ring opening and aryl hydroxylation (Scheme 1).
Iprodione Preparation Products And Raw materials
Raw materials
1of2
chevron_rightPreparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Pharmaffiliates Analytics and Synthetics P. Ltd | +91-172-5066494 | Haryana, India | 6739 | 58 | Inquiry |
| Pharma Affiliates | 172-5066494 | Haryana, India | 6754 | 58 | Inquiry |
| CLEARSYNTH LABS LTD. | +91-22-45045900 | Hyderabad, India | 6257 | 58 | Inquiry |
| Qingdao Trust Agri Chemical Co.,Ltd | +8613573296305 | China | 301 | 58 | Inquiry |
| Henan Bao Enluo International TradeCo.,LTD | +86-17331933971 +86-17331933971 | China | 2472 | 58 | Inquiry |
| HEBEI SHENGSUAN CHEMICAL INDUSTRY CO.,LTD | +8615350851019 | China | 1001 | 58 | Inquiry |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | China | 21630 | 55 | Inquiry |
| career henan chemical co | +86-0371-86658258 +8613203830695 | China | 29863 | 58 | Inquiry |
| Xiamen AmoyChem Co., Ltd | +86-86-5926051114 +8615060885618 | China | 6383 | 58 | Inquiry |
| Chongqing Chemdad Co., Ltd | +86-023-6139-8061 +86-86-13650506873 | China | 39894 | 58 | Inquiry |
36734-19-7(Iprodione)Related Search:
1of4
chevron_right




