PERCHLORIC ACID
- CAS No.
- 7601-90-3
- Chemical Name:
- PERCHLORIC ACID
- Synonyms
- NEOPRENE RUBBER;PERCHLORIC ACID 70%;Perchloric acid, ca. 70% Solution in Water;Perchloric acid, Puriss. p.a., ACS Reagent, 70% (Hg ?0.0000005%), packed in coated, shock- and leak-protected glass bottle;70-72%;GR,70-72%;Perchlorsure;PERCHLORIC ACID;Fraude's reagent;acideperchlorique
- CBNumber:
- CB4348487
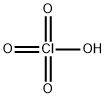
- Molecular Formula:
- ClHO4
- Molecular Weight:
- 100.46
- MOL File:
- 7601-90-3.mol
- MSDS File:
- SDS
- Modify Date:
- 2023/5/15 10:42:48
| Melting point | -18 °C |
|---|---|
| Boiling point | 203 °C |
| Density | 1.664 g/mL at 25 °C |
| vapor density | ~2.1 (vs air) |
| vapor pressure | 6.8 mm Hg ( 25 °C) |
| refractive index | 1.419 |
| Flash point | 104 °F |
| storage temp. | Flammables area |
| solubility | Water (Sparingly) |
| pka | -7[at 20 ℃] |
| form | Solution |
| color | APHA: ≤10 |
| Specific Gravity | approximate 1.54 |
| Odor | Odorless |
| PH | 0.1 (H2O, 20°C) |
| Water Solubility | Miscible with water. |
| Merck | 14,7153 |
| Stability | Stable. Avoid heat. May form explosive peroxides. Incompatible with a wide variety of substances, including organic materials, alcohols, amines, strong acids, strong bases, acid anhydrides, finely powdered metals, strong reducing agents. Contact with wood, paper and other celullose products may lead to explosion, as may contact with a vari |
| InChIKey | VLTRZXGMWDSKGL-UHFFFAOYSA-N |
| LogP | -4.62 |
| CAS DataBase Reference | 7601-90-3(CAS DataBase Reference) |
| NIST Chemistry Reference | Perchloric acid(7601-90-3) |
| EPA Substance Registry System | Perchloric acid (7601-90-3) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |     GHS03,GHS05,GHS07,GHS08 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H271-H290-H302-H314-H373 | |||||||||
| Precautionary statements | P210-P280-P301+P312-P303+P361+P353-P305+P351+P338-P314 | |||||||||
| Hazard Codes | C,O,Xi | |||||||||
| Risk Statements | 5-8-35-10-34-36/38 | |||||||||
| Safety Statements | 23-26-36-45-36/37/39 | |||||||||
| RIDADR | UN 2920 8/PG 2 | |||||||||
| WGK Germany | 1 | |||||||||
| RTECS | SC7500000 | |||||||||
| F | 3 | |||||||||
| Autoignition Temperature | 485 °C | |||||||||
| TSCA | Yes | |||||||||
| HS Code | 3822 00 00 | |||||||||
| HazardClass | 8 | |||||||||
| PackingGroup | II | |||||||||
| Toxicity | LD50 oral (rat) 1100 mg/kg LD50 oral (dog) 400 mg/kg |
|||||||||
| NFPA 704 |
|
PERCHLORIC ACID price More Price(40)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | 311421 | Perchloric acid 70%, 99.999% trace metals basis | 7601-90-3 | 50ML | ₹2045.93 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 77234 | Perchloric acid puriss. p.a., ACS reagent, packed in coated, shock- and leak-protected glass bottle, ≥60% (T) | 7601-90-3 | 1L | ₹11810.08 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 50439 | Perchloric acid concentrate 0.01?M HClO4 in water (0.01N), eluent concentrate for IC | 7601-90-3 | 1L | ₹5412.5 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 311421 | Perchloric acid 70%, 99.999% trace metals basis | 7601-90-3 | 250ML | ₹8995.58 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 311413 | Perchloric acid ACS reagent, 60% | 7601-90-3 | 100ML | ₹7555.85 | 2022-06-14 | Buy |
PERCHLORIC ACID Chemical Properties,Uses,Production
Chemical Properties
Perchloric acid, HCIO4, also known as Fraude's reagent,is a colorless, fuming,hygroscopic liquid that boils at 16°C(61OF). It is a strong oxidizer and is soluble in water. Cold dilute perchloric acid reacts with metals such as zinc and iron to yield hydrogen gas and the metallic perchlorate. Perchloric acid is used in electrolytic baths, electropolishing, explosives, analytical chemistry, and medicine.
Physical properties
Perchloric acid, HClO4, is a colorless liquid soluble in water. It is a strong acid comparable in strength to sulfuric and nitric acids. It is useful for preparing perchlorate salts, but it is also dangerously corrosive and readily forms explosive mixtures. Perchloric acid is produced by the treatment of sodium perchlorate with sulfuric acid and by the electrochemical oxidation of aqueous chlorine.
Uses
Perchloric acid salts are used as explosivesand in metal plating. They are also used as anoxidizer and as a reagent in chemical analysis. These salts are produced by distillingpotassium chlorate with concentrated H2SO4under reduced pressure..
General Description
A clear colorless odorless aqueous solution. Corrosive to metals and tissue. Closed containers may rupture violently under prolonged exposure to heat.
Air & Water Reactions
Water soluble with heat generation.
Reactivity Profile
PERCHLORIC ACID is a solution of a strong oxidizing acid. May react vigorously or deflagrate when mixed with oxidizable material [Merck]. This includes (but is not limited to) alcohols, amines, boranes, dicyanogen, hydrazines, hydrocarbons, hydrogen, nitroalkanes, powdered metals, silanes, or thiols [Bretherick 1979. p.174]. Perchloric acid ignites on contact with sulfinyl chloride. (Bailar, 1973, Vol. 2, 1442).
Health Hazard
Perchloric acid is a highly corrosive substance that causes severe burns on contact with the eyes, skin, and mucous membranes. The acute toxicity of perchloric acid is moderate. This substance is a severe irritant to the eyes, mucous membranes, and upper respiratory tract. Perchlorates are irritants to the body wherever they contact it. Perchloric acid has not been shown to be carcinogenic or to show reproductive or developmental toxicity in humans
Fire Hazard
Perchloric acid is noncombustible. The anhydrous (dehydrated) acid presents a serious explosion hazard. It is unstable and can decompose explosively at ordinary temperatures or in contact with many organic compounds.
Many heavy metal perchlorates and organic perchlorate salts are extremely sensitive explosives; the ammonium, alkali metal, and alkali earth perchlorates are somewhat less hazardous. Mixtures of perchlorates with many oxidizable substances are explosive.
Flammability and Explosibility
Perchloric acid is noncombustible. The anhydrous (dehydrated) acid presents a
serious explosion hazard. It is unstable and can decompose explosively at ordinary
temperatures or in contact with many organic compounds.
Many heavy metal perchlorates and organic perchlorate salts are extremely sensitive
explosives; the ammonium, alkali metal, and alkali earth perchlorates are somewhat
less hazardous. Mixtures of perchlorates with many oxidizable substances are
explosive.
Environmental Fate
Perchloric acid, in the presence of moisture, forms the negatively
charged perchlorate anion. The largest natural deposit of
perchlorate is located in Chile; the origin of the deposit is not
known.
Atmospheric perchlorate may be found near the sites where
it is manufactured and the locations where it is used. Accidental
spills of perchloric acid are another source of airborne
perchlorate. Perchlorate has a low vapor pressure and is not
found in the atmosphere as such; however, airborne particles
are known to be a source of perchlorate. The particles may fall
to the soil or be washed to the soil via rain. Soil particles
containing perchlorate can migrate in air currents or with
surface water or groundwater.
Perchlorate anions are highly mobile in groundwater
because of their charged state and because they adsorb to soil
particles poorly. Perchlorates in groundwater or surface water
are extremely persistent. They are extremely stable under
ambient conditions and tend not to react or degrade. Some
types of anaerobic bacteria are known to biodegrade perchlorate;
however, they are effective only under specific environmental
conditions (high levels of organic carbon and low levels
of oxygen and nitrate). Groundwater extraction is considered
inefficient for the removal of perchlorate.
Plants exposed to perchlorate in the soil moisture can also
take up perchlorate; some types of plants are known to
concentrate perchlorate.
storage
Splash goggles and rubber gloves should be worn when handling perchloric acid, and containers of the acid should be stored in a well-ventilated location separated from organic substances and other combustible materials. Work with >85% perchloric acid requires special precautions and should be carried out only by specially trained personnel.
Purification Methods
The 72% acid is been purified by double distillation from silver oxide under vacuum: this frees the acid from metal contamination. Distillation at atmospheric pressure is dangerous and explosive. The anhydrous acid is obtained by adding gradually 400-500mL of oleum (20% fuming H2SO4) to 100-120mL of 72% HClO4 in a reaction flask cooled in an ice-bath. The pressure is reduced to 1mm (or less), with the reaction mixture at 20-25o. The temperature is gradually raised during 2hours to 85o; the distillate is collected in a receiver cooled in Dry-ice. For further details of the distillation apparatus see Smith [J Am Chem Soc 75 184 1953]. It is HIGHLY EXPLOSIVE; a strong protective screen should be used at all times. [Schmeisser in Handbook of Preparative Inorganic Chemistry (Ed. Brauer) Academic Press Vol I pp 318-320 1963.]
Incompatibilities
Cold 70% perchloric acid is a strong acid but is not considered to be a strong oxidizing agent; however, more concentrated solutions are good oxidizers. Temperature increases the oxidizing power of perchloric acid, and hot concentrated solutions are very dangerous. Evaporation of a spill of the 70% solution may lead to the formation of more dangerous concentrations. Reaction of 70% perchloric acid with cellulose materials such as wood, paper, and cotton can produce fires and explosions. Oxidizable organic compounds including alcohols, ketones, aldehydes, ethers, and dialkyl sulfoxides can react violently with concentrated perchloric acid. All perchlorates are potentially hazardous when in contact with reducing agents.
Waste Disposal
Excess perchloric acid and waste material containing this substance should be placed in an appropriate container, clearly labeled, and handled according to your institution's waste disposal guidelines.
PERCHLORIC ACID Preparation Products And Raw materials
Raw materials
1of2
chevron_rightPreparation Products
1of4
chevron_right7601-90-3(PERCHLORIC ACID)Related Search:
1of4
chevron_right




