CHLORIC ACID
- CAS No.
- 7790-93-4
- Chemical Name:
- CHLORIC ACID
- Synonyms
- C01485;NA-9191;chloric;NA 2626;Hypure C;chloricacid;Chloride Acid;Chloric acid solution;CHLORIC ACID, 35 WT. % SOLUTION;CHLORIC ACID ISO 9001:2015 REACH
- CBNumber:
- CB0497587
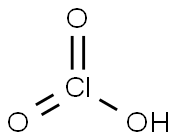
- Molecular Formula:
- ClHO3
- Molecular Weight:
- 84.46
- MOL File:
- 7790-93-4.mol
- MSDS File:
- SDS
- Modify Date:
- 2023/10/17 17:11:54
| Melting point | <-20℃ [CRC10] |
|---|---|
| Boiling point | >100 °C |
| Density | 1.2 g/mL at 25 °C |
| solubility | very soluble in H2O |
| color | exists only in aqueous solution |
| Water Solubility | very soluble H2O [CRC10] |
| Merck | 13,2109 |
| CAS DataBase Reference | 7790-93-4 |
| EPA Substance Registry System | Chloric acid (7790-93-4) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS03,GHS05 |
|---|---|
| Signal word | Danger |
| Hazard statements | H314-H271 |
| Precautionary statements | P260-P264-P280-P301+P330+P331-P303+P361+P353-P363-P304+P340-P310-P321-P305+P351+P338-P405-P501-P210-P220-P221-P280-P283-P306+P360-P371+P380+P375-P370+P378-P501 |
| Hazard Codes | O,C |
| Risk Statements | 8-34 |
| Safety Statements | 17-26-36/37/39-45 |
| RIDADR | 2626 |
| HazardClass | 5.1 |
| PackingGroup | II |
CHLORIC ACID Chemical Properties,Uses,Production
Description
Chloric acid, HClO3, is an oxoacid of chloride, and
the formal precursor of chlorate salts. It is a strong
acid (pKa=-1) and a strong oxidizing agent. It is stable
in cold aqueous solution up to a concentration of
approximately 30%, and solution of up to 40% can be
prepared by careful evaporation under reduced pressure.
Above these concentrations, and on warming,
chloric acid solutions decompose to give a variety of
products, for example:
8HClO3→4HClO4+2H2O+2Cl2+3O2
Note that it disproportionates into perchloric acid
in which the chlorine atom has a+7 oxidation state
and also the zero oxidation state of chlorine gas (an
oxidation–reduction reaction). Thus, under the proper
conditions, it can also be used to make perchloric acid.
3HClO3→HClO4+H2O+2ClO2
Chemical Properties
can occur only in an aqueous solution; oxidizing agent; preparation: reaction between H2SO4 and barium chlorate; used as a catalyst in the polymerization of acrylonitrile, as an oxidizing agent [MER06] [HAW93]
Uses
Oxidizing agent; with H2SO3 as catalyst in acrylonitrile polymerization.
General Description
CHLORIC ACID is a colorless liquid. CHLORIC ACID will accelerate the burning of combustible materials and can ignite most on contact. CHLORIC ACID is corrosive to metals and tissue. CHLORIC ACID is used as a reagent in chemical analysis and to make other chemicals.
Air & Water Reactions
Water soluble.
Reactivity Profile
Self-reactive. Concentrations of CHLORIC ACID above 40% decompose [Mellor 2 Supp. 1:576 1956]. Antimony sulfide and concentrated solutions of CHLORIC ACID react with incandescence [Mellor Supp. II Part I:584 1956]. Arsenic sulfide and concentrated solutions of CHLORIC ACID react with incandescence . Reacts with vigor even explodes with other metal sulfides, i.e. copper sulfide [Mellor Supp. II Part I:584 1956]. In contact with oxidizable materials, including ammonia, reactions can be extremely violent. Filter paper ignites after soaking in CHLORIC ACID, [Mellor, 1946, Vol. 2, 310]. Explosions have been recorded by mixtures of CHLORIC ACID solution with metals such as: antimony, bismuth, and iron. This is due to the formation of explosive compounds including hydrogen.
Hazard
Toxic by ingestion and inhalation. Strong oxidizer, ignites organic materials on contact.
Health Hazard
Inhalation, ingestion or contact (skin, eyes) with vapors or substance may cause severe injury, burns or death. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may cause pollution.
Fire Hazard
These substances will accelerate burning when involved in a fire. Some may decompose explosively when heated or involved in a fire. May explode from heat or contamination. Some will react explosively with hydrocarbons (fuels). May ignite combustibles (wood, paper, oil, clothing, etc.). Containers may explode when heated. Runoff may create fire or explosion hazard.
Safety Profile
A poison. A strong irritant by ingestion and inhalation. Dangerous fire hazard; ignites organic matter upon contact. A very powerful oxidizing agent. Violent or explosive reaction with oxidlzable materials. Aqueous solutions decompose explosively during evaporation. Solutions greater than 40% are unstable. Reacts violently with NH3, Sb, Sb2S3, AsS3, Bi, CuS, PHI4+ SnS2, SnS. Reaction with cellulose causes ignition after a delay period. Dangerous reaction with metal sulfides and metal chlorides (e.g., incandescent reaction with antimony trisulfide, arsenic trisulfide, tin(lI)sulfide, tin(Iv> sulfide, explosion on contact with copper sulfide). Reaction with metals (e.g., antimony, bismuth, iron) forms explosive products. When heated to decomposition it emits toxic fumes of Cl-. See also CHLORATES and CHLORINE.
CHLORIC ACID Preparation Products And Raw materials
Raw materials
Preparation Products
1of4
chevron_right| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Chongqing Chemdad Co., Ltd | +86-023-6139-8061 +86-86-13650506873 | China | 39916 | 58 | Inquiry |
| Shaanxi Dideu Medichem Co. Ltd | +86-029-89586680 +86-18192503167 | China | 9030 | 58 | Inquiry |
| Shaanxi DIDU pharmaceutical and Chemical Co., Ltd | 17691182729 18161915376 | China | 10011 | 58 | Inquiry |
| Guangdong wengjiang Chemical Reagent Co., Ltd. | 0751-2815987 13927875076 | China | 9977 | 58 | Inquiry |
| Yick-Vic Chemicals & Pharmaceuticals (HK) Ltd | (852)2541 2772 | China | 6214 | 58 | Inquiry |
| kemikalieimport | + 45 - 2034 3359 | Europe | 6699 | 47 | Inquiry |
| ecochem international chemical broker | +45 45 42 34 36 | Europe | 6385 | 66 | Inquiry |
| HONEST JOY HOLDINGS LIMITED | +86-755-26404303 | United States | 6702 | 54 | Inquiry |
| SIGMA-RBI | 800 736 3690 (Orders) | Switzerland | 6913 | 91 | Inquiry |
| Riedel-de Haen AG | 800 558-9160 | United States | 6825 | 87 | Inquiry |
7790-93-4(CHLORIC ACID)Related Search:
1of4
chevron_right




