Cobalt oxide
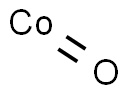
- CAS No.
- 1307-96-6
- Chemical Name:
- Cobalt oxide
- Synonyms
- CoO;COBALT(II) OXIDE;COBALTOUS OXIDE;Cobalt oxide (CoO);1,2-Benzenedimethanethiol, 4,5-dimethyl-;Zaffre;ci77322;Black 13;c.i.77322;Oxocobalt
- CBNumber:
- CB9216703
- Molecular Formula:
- CoO
- Molecular Weight:
- 74.93
- MOL File:
- 1307-96-6.mol
- MSDS File:
- SDS
- Modify Date:
- 2024/5/29 14:11:26
| Melting point | 1785 °C |
|---|---|
| Density | 6.45 |
| vapor pressure | 0Pa at 20℃ |
| solubility | insoluble in H2O; soluble in acid solutions |
| form | Powder |
| Specific Gravity | 6.45 |
| color | Green-brown |
| Water Solubility | insoluble |
| Sensitive | Air Sensitive |
| Merck | 14,2446 |
| Exposure limits |
ACGIH: TWA 1 mg/m3 OSHA: TWA 15 mg/m3; TWA 5 mg/m3 |
| Stability | Stability Stable, but may be moisture sensitive. |
| InChI | InChI=1S/Co.O |
| InChIKey | IVMYJDGYRUAWML-UHFFFAOYSA-N |
| SMILES | O=[Co] |
| CAS DataBase Reference | 1307-96-6(CAS DataBase Reference) |
| NIST Chemistry Reference | Cobalt monoxide(1307-96-6) |
| EPA Substance Registry System | Cobalt(II) oxide (1307-96-6) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS06,GHS08,GHS09 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H301-H317-H330-H334-H350i-H360F-H410 | |||||||||
| Precautionary statements | P202-P260-P273-P280-P302+P352-P304+P340+P310 | |||||||||
| Hazard Codes | Xn,N,T+ | |||||||||
| Risk Statements | 22-43-50/53-42/43-26 | |||||||||
| Safety Statements | 24-37-60-61-45-36/37-28 | |||||||||
| RIDADR | UN 3288 6.1/PG 3 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | GG2800000 | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 6.1(b) | |||||||||
| PackingGroup | III | |||||||||
| HS Code | 28220000 | |||||||||
| Toxicity | LD50 orally in rats: 1.70 g/kg (Smyth) | |||||||||
| NFPA 704 |
|
Cobalt oxide price More Price(22)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | 529443 | Cobalt(II) oxide 99.99% trace metals basis | 1307-96-6 | 5G | ₹22007.23 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 529443 | Cobalt(II) oxide 99.99% trace metals basis | 1307-96-6 | 25G | ₹53800.25 | 2022-06-14 | Buy |
| Sigma-Aldrich | 343153 | Cobalt(II) oxide ?325?mesh | 1307-96-6 | 10G | ₹7523.38 | 2022-06-14 | Buy |
| Sigma-Aldrich | 343153 | Cobalt(II) oxide ?325?mesh | 1307-96-6 | 10G | ₹7523.38 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 343153 | Cobalt(II) oxide ?325?mesh | 1307-96-6 | 10G | ₹7523.38 | 2022-06-14 | Buy |
Cobalt oxide Chemical Properties,Uses,Production
Description
This is obtained as an olive-green powder by heating the metal in air or steam or by thermal decomposition of the hydroxide, carbonate or nitrate. It has the sodium chloride lattice and is antiferromagnetic below 292°K. When heated in oxygen above 400° the black oxide Co3O4 is obtained. This oxide is isomorphous with magnetite Fe3O4, and has tetrahedrally surrounded cobalt(II) ions and octahedrally surrounded cobalt(III) ions. Both these oxides are readily reduced to the metal by heating in hydrogen or with carbon. The reactions of CoO with silica, alumina and zinc oxide are used to form pigments in the ceramic industry.
Chemical Properties
Cobalt oxide is a high-valent oxide of cobalt, with a theoretical cobalt content of 71.06% and an oxygen content of 28.94%, which is a black amorphous powder. Cobalt oxide is a kind of unstable and impossible free state compound, usually refers to the cobalt oxide are attached with a certain amount of cobalt tetraoxide when heated cobalt oxide is reduced to cobalt tetraoxide. The theoretical cobalt content of cobalt tetraoxide is 73.43% and the oxygen content is 26.57%, with the appearance of gray-black or black powder. 
The cobalt oxide used in batteries is cobalt tetraoxide, which has a spinel structure. It can be used as anode material for lithium-ion batteries and is also the main raw material for the preparation of lithium cobaltate, the cathode material for lithium-ion batteries.
Physical properties
The commercial product is usually dark grey powder, but the color may vary from olive geeen to brown depending on particle size; density 6.44 g/cm3, which also may vary between 5.7 to 6.7 g/cm3, depending on the method of preparation; melts around 1,830°C; insoluble in water; soluble in acids and alkalis.
Uses
In pigments for ceramics; glass coloring and decolorization; oxidation catalyst for drying oils, fast-drying paints and varnishes; preparation of cobalt-metal catalysts, Co powder for binder in sintered tungsten carbide; in semiconductors.
Structure and conformation
Green cobalt(II) oxide (CoO) has the rock salt (NaCl) structure. It is easily oxidized with water and oxygen to brown cobalt(III) hydroxide (Co(OH)3). At temperatures of 600℃-700℃, CoO oxidizes to the blue cobalt (II,III) oxide (Co3O4), which has a spinel structure. Black cobalt(III) oxide (Co2O3) also exists. Cobalt oxides are antiferromagnetic at low temperature: CoO (Ne′el temperature 18℃/291K) and Co3O4 (Ne′el temperature: 2233℃/40K), which is analogous to magnetite (Fe3O4), with a mixture of 12 and 13 oxidation states.
Cobalt oxide Preparation Products And Raw materials
Raw materials
1of3
chevron_rightPreparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| GLR Innovations | +91 9891111994 | New Delhi, India | 4542 | 58 | Inquiry |
| ADVENT CHEMBIO PRIVATE LIMITED | +91-9821524116 +91-9821524116 | Mumbai, India | 353 | 58 | Inquiry |
| Yogi Dye Chem Industries | +91-9821098577 +91-9821098577 | Mumbai, India | 124 | 58 | Inquiry |
| Rivashaa Agrotech Biopharma Pvt. Ltd. | +91-26463395 +91-7926462688 | Gujarat, India | 1615 | 58 | Inquiry |
| S.V ENTERPRISES | +919322701159 | Mumbai, India | 150 | 58 | Inquiry |
| PERFECT CHEMICAL | +91-9820465461 +91-9820465461 | Mumbai, India | 391 | 58 | Inquiry |
| P K Chlorochem Pvt. Ltd. | 91-265-2633297 | Gujarat, India | 88 | 58 | Inquiry |
| S.K. CHEMICAL INDUSTRIES | +91-22-66370299 Ext 21 / 91-22-23437380 Ext 25 | New Delhi, India | 262 | 30 | Inquiry |
| Advent Chembio Pvt., Ltd. | 91-22-27690836 | Maharashtra, India | 625 | 58 | Inquiry |
| Qualikems Fine Chem Pvt., Ltd. | 91-265-2841531 | Gujarat, India | 54 | 58 | Inquiry |
1307-96-6(Cobalt oxide )Related Search:
1of4
chevron_right




