Barium
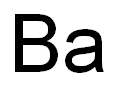
- CAS No.
- 7440-39-3
- Chemical Name:
- Barium
- Synonyms
- bario;BARIUM;barium(0);BARIUM, ROD;BARIUM METAL;Barium, 99+%;Barium powder;Barium element;bario (spanish);baryum (french)
- CBNumber:
- CB0854186
- Molecular Formula:
- Ba
- Molecular Weight:
- 137.33
- MOL File:
- 7440-39-3.mol
- MSDS File:
- SDS
- Modify Date:
- 2024/5/29 13:19:41
| Melting point | 725 °C(lit.) |
|---|---|
| Boiling point | 1640 °C(lit.) |
| Density | 3.6 g/mL at 25 °C(lit.) |
| storage temp. | water-free area |
| solubility | reacts with H2O; slightly soluble in ethanol |
| form | rod |
| Specific Gravity | 3.51 |
| color | Silver-gray |
| Resistivity | 50.0 μΩ-cm, 20°C |
| Water Solubility | soluble with H2 evolution in cold H2O and hot H2O; slightly soluble alcohol; insoluble benzene [CRC10] |
| Sensitive | air sensitive, moisture sensitive |
| Merck | 13,967 |
| Exposure limits | TLV-TWA 0.5 mg/m3 (for soluble compounds) (ACGIH and MSHA); IDLH (for soluble compounds) 250 mg/m3 (NIOSH). . |
| Stability | Stability Reacts vigorously or violently with acids, water, tetrachloromethane, small halogenated hydrocarbons. Should be stored under an inert material such as petroleum ether to exclude air. Flammable. |
| CAS DataBase Reference | 7440-39-3(CAS DataBase Reference) |
| EPA Substance Registry System | Barium (7440-39-3) |
| Modulus of Elasticity | 12.8 GPa |
|---|---|
| Poissons Ratio | 0.28 |
| Shear Modulus | 5.00 GPa, Calculated |
| Bulk Modulus | 10.2 GPa |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS02,GHS05,GHS06 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H228-H260-H301-H314 | |||||||||
| Precautionary statements | P210-P231+P232-P260-P280-P303+P361+P353-P304+P340+P310-P305+P351+P338 | |||||||||
| Hazard Codes | C,Xi,F | |||||||||
| Risk Statements | 25-26-34-36/37/38-14/15-11 | |||||||||
| Safety Statements | 23-26-36-36/37/39-45-43-36/37-16 | |||||||||
| RIDADR | UN 3264 8/PG 3 | |||||||||
| OEB | C | |||||||||
| OEL | TWA: 0.5 mg/m3 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | CQ8370000 | |||||||||
| TSCA | Yes | |||||||||
| HS Code | 2805 19 10 | |||||||||
| HazardClass | 8 | |||||||||
| PackingGroup | III | |||||||||
| Toxicity | An element; the heaviest of the stable alkaline earths. Barium sulfate is used as a diagnostic aid in radiology due to its radio-opaqueness and, because of its insolubility and lack of absorption, it is safe barring iatrogenic episodes. Poisoning usually results from deliberate or accidental ingestion of soluble barium compounds. The Ba2+ ion is a muscle poison due to the blocking of the K1 channels of the Na+/K+ pump in cell membranes. Because cases of barium poisoning are accompanied by severe hypokalemia, potassium infusion is an effective antidote. The toxicity of barium compounds depends on their solubility, with the free ion being readily absorbed from gastrointestinal tract or lung, whereas the sulfate is essentially unabsorbed. Thus, administration of soluble sulfates immediately after ingestion is another effective antidote. | |||||||||
| IDLA | 50 mg Ba/m3 | |||||||||
| NFPA 704 |
|
Barium price More Price(15)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | 735787 | Barium beads, 0.5-2.0?mm particle size, 99% trace metals basis | 7440-39-3 | 5G | ₹4198.9 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 474711 | Barium dendritic pieces, purified by distillation, 99.99% trace metals basis | 7440-39-3 | 5G | ₹37937.8 | 2022-06-14 | Buy |
| Sigma-Aldrich | 474711 | Barium dendritic pieces, purified by distillation, 99.99% trace metals basis | 7440-39-3 | 25G | ₹137582.55 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 474711 | Barium dendritic pieces, purified by distillation, 99.99% trace metals basis | 7440-39-3 | 25G | ₹137582.55 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 441880 | Barium dendritic pieces, purified by distillation, 99.9% trace metals basis | 7440-39-3 | 5G | ₹18240.95 | 2022-06-14 | Buy |
Barium Chemical Properties,Uses,Production
Chemical Properties
The chemical process of Barium is similar to Ca and Sr, but it is stronger. The single crystal of oxide BaO is easy to grow, and the absorption coefficient and the reflectance spectrum show the peaks at 3.77, 3.9, 4.0, 4.3 and 6 eV.
Chemical Properties
Barium is a silvery-white metal. It exists in nature only in ores containing mixtures of elements. The important combinations are peroxide, chloride, sulfate, carbonate, nitrate, and chlorate. The pure metal oxidizes readily and reacts with water, emitting hydrogen. It combines with other chemicals such as sulfur or carbon and oxygen to form barium compounds. Barium compounds are used by the oil and gas industries to make drilling muds. Barium attacks most metals with the formation of alloys; iron is the most resistant to alloy formation. Barium forms alloys and intermetallic compounds with lead, potassium, platinum, magnesium, silicon, zinc, aluminum, and mercury. Barium compounds exhibit close relation-ships with the compounds of calcium and strontium, which are also alkaline earth metals. Doctors sometimes use barium sulfate to perform medical tests and to take x-rays of the gastrointestinal tract. Twentyive barium isotopes have been identii ed. 138Ba is the most abundant; the others are unstable isotopes with half-lives ranging from 12.8 days for 140Ba to 12 sec for 143Ba. Two of these isotopes, 131Ba and 139Ba, are used in research as radioactive tracers. The general population is exposed to barium through air, drinking water, and food.
Physical properties
Barium is the fifth element in group 2 (IIA) of the alkali earth metals and has most of theproperties and characteristics of the other alkali earth metals in this group. For example, theyall are called alkaline earths because, when first discovered, they exhibited both characteristicsof alkaline (basic) substances and characteristics of the earth from which they came. Ancienthumans did not know they were metals because their metallic forms do not exist in nature.Barium is a silvery metal that is somewhat malleable and machineable (can be worked on alathe, stretched and pounded). Its melting point is 725°C, its boiling point is about 1640°C,and its density is 3.51 g/cm3. (The accurate figures for its properties are difficult to determinebecause of barium’s extreme activity—the pure metal will ignite when exposed to air, water,ammonia, oxygen, and the halogens.
Isotopes
Naturally occurring barium is a mixture of seven stable isotopes: barium-138 (71.66%), barium-137 (11.32%), barium-136 (7.81%), barium-135 (6.59%), barium-134 (2.42%), barium-130 (0.101%), and barium- 132 (0.097%). About six times this many radioactive isotopes have been prepared with mass numbers ranging from 114 to 153. Of the 40 isotopes known, most are highly radioactive and have half-lives in the several milliseconds to a few days range. The only notable exceptions are 133Ba with a half-life of 10.51 years, 128Ba (2.43 days), 141Ba (11.50 days) and 140Ba (12.75 days).
Origin of Name
The name barium is derived from the Latin word barys, which means “heavy.
Occurrence
Barium is the 17th most abundant element in the Earth’s crust, making up about 0.05%of the crust. It is found in the minerals witherite, which is barium carbonate (BaCO3), andbarite, known as barium sulfate (BaSO4). Pure barium metal does not exist on Earth—only ascompounds or in minerals and ores. Barium ores are found in Missouri, Arkansas, Georgia,Kentucky, Nevada, California, Canada, and Mexico.It is produced by the reduction of barium oxide (BaO), using aluminum or silicon ina high-temperature vacuum. It is also commercially produced by the electrolysis of moltenbarium chloride (BaCl2) at about 950oC, wherein the barium metal is collected at the cathodeand chlorine gas is emitted at the anode.
Characteristics
When barium burns in air, it produces barium oxide (2Ba + O2 → 2BaO). When metallicbarium burns in water, it forms barium hydroxide [Ba + 2H2O → Ba(OH)2 + H2↑]. Severalbarium compounds burn with a bright green flame, which make them useful for fireworks.Barium is more reactive with water than are calcium and strontium. This is a result of thevalence electrons’ being further from the positive nucleus. Therefore, barium is more electronegative than the alkali earth metals with smaller nuclei.In powdered form, it will burst into a bright green flame at room temperature.
Uses
Pure barium metal has few commercial uses because of it reactivity with air and water.Nevertheless, this property makes it useful as a “getter” or scavenger to remove the last tracesof gas from vacuum tubes. Barium metal is used to form alloys with other metals. One alloy isused to make sparkplugs that easily emit electrons when heated, thus improving the efficiencyof internal combustion engines.Its compounds have many practical uses. For example, when the mineral barite is groundup into a fine powder, it can be used as a filler and brightener for writing and computer paper.It is also used (along with zinc sulfide) as a pigment, called lithopone, for white paint. Bariumcompounds are also used in the manufacture of plastics, rubber, resins, ceramics, rocket fuel,fireworks, insecticides, and fungicides and to refine vegetable oils.A major medical use is a solution of barium sulfide (with flavoring) that is ingested bypatients undergoing stomach and intestinal X-ray and CT scan examinations. Barium sulfideis opaque to X-rays, and thus it blocks the transmission of the rays. The organs appear in contrast against a background, which highlights any problems with the digestive system.
Production Methods
The minerals are BaSO4 and BaCO3. Metal barium is obtained by reducing the barium oxide at a high temperature in vacuum with Al or Si or by doing electrolysis with the Hg cathode and evaporating Hg of amalgam formed at the process slowly to segregate Ba. To obtain vacuum evaporated films, direct heating with the conical basket of W, Ta, Mo, Nb, Ni, Fe, chromel, etc., or with the boat of Ta, Mo is used. Ba reacts with alumina. The rate of evaporation is 2.28×10-4 g/cm2 s with the evaporation temperature of 629 ℃.
Definition
A dense, low-melting reactive metal; the fifth member of group 2 (formerly IIA) of the periodic table and a typical alkaline-earth element. The electronic configuration is that of xenon with two additional outer 6s electrons. Barium is of low abundance; it is found as witherite (BaCO3) and barytes (BaSO4). The metal is obtained by the electrolysis of the fused chloride using a cooled cathode which is slowly withdrawn from the melt. Because of its low melting point barium is readily purified by vacuum distillation. Barium metal is used as a ‘getter’, i.e., a compound added to a system to seek out the last traces of oxygen; and as an alloy constituent for certain bearing metals.
Barium has a low ionization potential and a large radius. It is therefore strongly electropositive and its properties, and those of its compounds, are very similar to those of the other alkaline-earth elements calcium and strontium.
General Description
Barium alloy, pyrophoric is mixture of barium and other metals or nonmetallic elements to improve the specific usefulness of barium. Barium alloys are a solid and can ignite spontaneously in contact with air. Barium is toxic and products given off in fire could be very toxic.
Air & Water Reactions
Finely divided metal powder is pyrophoric, ignites spontaneously in air [Bretherick 1979 p. 170-171]. Alloys containing a substantial proportion of barium rapidly decomposed water. The heat of the reaction is sufficient that the evolved hydrogen may ignite [Lab. Govt. Chemist 1965].
Reactivity Profile
Alloys containing a substantial amount of barium react violently with acids [Lab. Gov. Chemist 1965].
Hazard
Barium metal, in powder form, is flammable at room temperature. It must be stored in anoxygen-free atmosphere or in petroleum.
Many of barium’s compounds are toxic, especially barium chloride, which affects the functioningof the heart, causing ventricular fibrillation, an erratic heartbeat that can lead to death.Several of barium’s compounds are explosive as well as toxic if ingested or inhaled. Care shouldbe used when working with barium and other alkali metals in the laboratory or in industry.
Fire Hazard
Flammable/combustible material. May ignite on contact with moist air or moisture. May burn rapidly with flare-burning effect. Some react vigorously or explosively on contact with water. Some may decompose explosively when heated or involved in a fire. May re-ignite after fire is extinguished. Runoff may create fire or explosion hazard. Containers may explode when heated.
Industrial uses
Barium (symbol Ba) is a metallic element thatoccurs in combination in the minerals witheriteand barite, which are widely distributed. Themetal is silvery white in color and can beobtained by electrolysis from the chloride, butit oxidizes so easily that it is difficult to obtainin the metallic state. Its melting point is 850°C,and its specific gravity 3.78. The most extensiveuse of barium is in the form of its compounds.The salts that are soluble, such as sulfide andchloride, are toxic. An insoluble, nontoxic bariumsulfate salt is used in radiography. Bariumcompounds are used as pigments, in chemicalmanufacturing, and in deoxidizing alloys of tin, copper, lead, and zinc. Barium is introducedinto lead-bearing metals by electrolysis toharden the lead.Barium is also a key ingredient in ceramicsuperconductors.
Safety Profile
Water and stomach acids solubilize barium salts and can cause poisoning. Symptoms are vomiting, colic, diarrhea, slow irregular pulse, transient hypertension, and convulsive tremors and muscular paralysis. Death may occur in a few hours to a few days. Half-life of barium in bone has been estimated at 50 days. Dust is dangerous and explosive when exposed to heat, flame, or chemical reaction. Violent or explosive reaction with water, CCh, fluorotrichloromethane, trichloroethylene, and C2Cl4. Incompatible with acids, C2CLF3, C2H2FCl3, C2HCl3 and water, 1,1,2- trichlorotrifluoroethane, and fluorotrichloroethane. The powder may ignite or explode in air or other oxidizing gases. See also BARIUM COMPOUNDS.
Potential Exposure
Metallic barium is used for removal of residual gas in vacuum tubes and in alloys with nickel, lead, calcium, magnesium, sodium, and lithium. Barium compounds are used in the manufacture of lithopone (a white pigment in paints), chlorine, sodium hydroxide, valves, and green flares; in synthetic rubber vulcanization; X-ray diagnostic work, glassmaking, papermaking, beet-sugar purification; animal and vegetable oil refining. They are used in the brick and tile, pyrotechnics, and electronics industries. They are found in lubricants, pesticides, glazes, textile dyes and finishes; pharmaceuticals; in cements which will be exposed to saltwater; and barium is used as a rodenticide, a flux for magnesium alloys, a stabilizer and mold lubricant in the rubber and plastics industries, an extender in paints; a loader for paper, soap, rubber, and linoleum; and as a fire extinguisher for uranium or plutonium fires.
Environmental Fate
Ingestion of toxic doses of barium affects the muscles, especially the heart. Barium has a digitalis-type effect on the heart. Ventricular fibrillation and slowed pulse rate are noted. This may be related to barium’s tendency to displace potassium; the resulting potassium deficiency causes muscle weakness.
Shipping
UN1400 Barium, Hazard Class: 4.3; Labels: 4.3—Dangerous when wet material. UN1854 Barium alloys, pyrophoric, Hazard Class: 4.2; Labels: 4.2—Spontaneously combustible material. UN1564 Barium compound, n.o.s., Hazard Class: 6.1; Labels: 6.1—Poisonous materials.
Purification Methods
Barium is cleaned by washing with diethyl ether to remove adhering paraffin, then filed in an argon-filled glove box, washed first with ethanol containing 2% conc HCl, then with dry ethanol. It is dried in a vacuum and stored under argon [Addison et al. J Chem Soc 3868 1962]. It has also been purified by double distillation under 10mm of argon pressure.
Structure and conformation
The space lattice of Barium belongs to the cubic system, and its body-centered cubic lattice has a lattice constant of a=0.5009 nm.
Incompatibilities
Barium powder may spontaneously ignite on contact with air. It is a strong reducing agent and Barium 337 reacts violently with oxidizers and acids. Reacts with water, forming combustible hydrogen gas and barium hydroxide. Reacts violently with halogenated hydrocarbon solvents, causing a fire and explosion hazard.
Waste Disposal
Barium in solution (see spill handling) may be precipitated with soda ash and the sludge may be landfilled.
Barium Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| CLEARSYNTH LABS LTD. | +91-22-45045900 | Hyderabad, India | 6351 | 58 | Inquiry |
| Alfa Aesar | 1 800 209 7001 | Maharashtra, India | 6913 | 58 | Inquiry |
| Asian Drill Chem Solutions | 07942559715 | Ahmedabad, India | 8 | 58 | Inquiry |
| Chemi Enterprises LLP | 08048951645 | Mumbai, India | 14 | 58 | Inquiry |
| S. V. Plastochem Pvt., Ltd. | 91-253-2597815 | Maharashtra, India | 11 | 58 | Inquiry |
| Venus Dye Chem | 91-9924061717 | Gujarat, India | 63 | 58 | Inquiry |
| Om Industrial Services | 08048619886 | Vadodara, India | 4 | 58 | Inquiry |
| Indian Overseas Corporation. | 91-22-66335111 | Maharashtra, India | 20 | 58 | Inquiry |
| ALPHA CHEMIKA | 08048602756 | Mumbai, India | 76 | 58 | Inquiry |
| Arnish Laborates Private Limited | 08048957357 | Mumbai, India | 204 | 58 | Inquiry |
| Supplier | Advantage |
|---|---|
| CLEARSYNTH LABS LTD. | 58 |
| Alfa Aesar | 58 |
| Asian Drill Chem Solutions | 58 |
| Chemi Enterprises LLP | 58 |
| S. V. Plastochem Pvt., Ltd. | 58 |
| Venus Dye Chem | 58 |
| Om Industrial Services | 58 |
| Indian Overseas Corporation. | 58 |
| ALPHA CHEMIKA | 58 |
| Arnish Laborates Private Limited | 58 |
Related articles
- Barium: Source and Preparation
- The abundance of barium is 0.0425% in the Earth’s crust. The main commercial source of barium is baryte (also called barite, b....
- May 29,2024
- Exploring the Significance of Barium Chemistry in Soil Distribution and Plant Uptake
- Barium chemistry studies its reactivity, distribution in soils, uptake by plants, and environmental impact for industrial and ....
- Mar 27,2024
- Barium: Discovery, Sources and the Use in Fireworks
- Barium is a soft silvery metal, always found combined with other elements. Barium emits a green flame when it burns, which is ....
- Jan 24,2024
7440-39-3(Barium)Related Search:
1of4
chevron_right




