水酸化ナトリウム 化学特性,用途語,生産方法
外観
無色澄明の液体
定義
本品は、ナトリウムの水酸化物であり、次の化学式で表される。
溶解性
水又はエタノール(95)に溶けやすく、ジエチルエーテルにほとんど溶けない。
解説
純粋なものは無色透明な固体で,融点328 ℃.通常は少量の水と炭酸塩などを含む白色のもろい固体で,融点318.4 ℃,沸点1390 ℃.密度2.13 g cm-3.潮解性で,水には多量の熱を発して溶ける.水100 g に対する溶解度は42 g(0 ℃),109 g(20 ℃),347 g(100 ℃).エタノールに易溶,エーテル,アセトンに不溶.水溶液は強アルカリ性で濃厚なものは腐食性が強く,有機物を分解し皮膚をおかす.とくに眼に触れると失明のおそれがある.
森北出版「化学辞典(第2版)
用途
製薬、製剤原料。
用途
酸の定量(容量分析)
用途
試液調製、精密な酸中和剤(濃度が高すぎ、通常の容量分析にはほとんど用いられない)。
用途
アミノ酸自動分析用調製液製造原料及び試料前処理試薬。
用途
食品添加物。
用途
一般分析用試液
用途
化学繊維?紙?パルプ製造用,有機薬品?無機薬品?医薬?農薬?染料中間体製造用,グルタミン酸ソーダ原料,食品製造用
製造
塩化ナトリウム水溶液を隔膜を用いて電解すると陰極室に生成する(隔膜法).また,炭酸ナトリウムと水酸化カルシウムとの反応によっても得られる水酸化ナトリウム.
化粧品の成分用途
pH調整剤、変性剤
効能
アルカリ化剤, 腐食剤
特徴
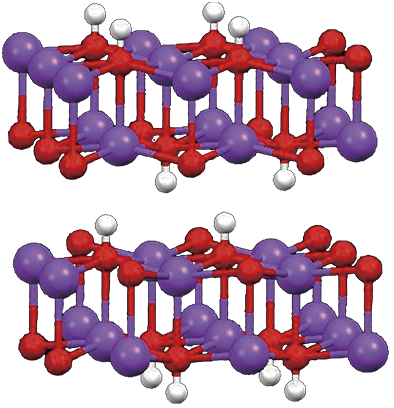
Na+の周りにOH-がピラミッドをつくっている.Na-O(頂点)232.5 pm,Na-O(底面)242.5 pm,∠NaO(頂点)H 180˚
主な用途/役割
ユリア樹脂系接着剤、メラミン樹脂系接着剤、フェノール樹脂系接着剤の触媒として使用される。
商品名
水酸化ナトリウム (小堺製薬)
使用上の注意
空気中で速やかに二酸化炭素を吸収する。湿気によって潮解する。
化学的特性
Sodium hydroxide, NaOH,also referred to as caustic soda or sodium hydrate(and formerly known as lye), is a white,massive, deliquescent crystalline solid that is soluble in water,alcohol, and glycerol. It melts at 318°C (606 OF) and is the most widely used and available alkaline chemical. Most sodium hydroxide is produced as a coproduct of chlorine through the use of electrolytic cells;the cells are of the diaphragm, mercury, or membrane type. Some sodium hydroxide is marked as produced in the cells;most is evaporated and sold as 50% and 73% solutions or as anhydrous beads. Most caustic end uses require solutions of relatively low concentrations. Caustic soda is used as an analytical reagent and chemical intermediate, in scouring and cleaning baths,in rubber reclaiming and petroleum refining, in quenching baths for heat treating of steel,in cutting and soluble oils,in soaps and detergents, and in a wide variety of other applications.
物理的性質
White orthorhombic crystals, produced in the form of pellets, lumps, sticks, beads, chips, flakes or solutions; hygroscopic; very corrosive; rapidly absorbs CO2 and water from the air; density 2.13 g/cm
3; melts at 323°C; vaporizes at 1388°C; vapor pressure 1 torr at 739°C and 5 torr at 843°C; very soluble in water (110 g/100mL at room temperature), generating heat on dissolution; aqueous solutions highly alkaline, pH of 0.5% solution about 13 and 0.05% solution about 12; soluble in methanol, ethanol and glycerol (23.8 g/100 mL methanol and 13.9 g/100 mL ethanol at ambient temperatures.).
使用
Sodium hydroxide is one of the most important industrial chemicals. In volume, it is in the top ten chemicals produced in the United States. It is used in manufacturing a large number of compounds including several sodium salts, in treating cellulose for producing rayon and cellophane, and in manufacturing soaps, detergents, pulp, and paper. Sodium hydroxide is a common neutralizing agent for acids in acid-base titrations and petroleum refining. Another major application is extracting metals from their ores where alkali fusion, such as fusion with caustic soda, often is applied to open the ores. Additionally, sodium hydroxide is used to precipitate metals as hydroxides. Other uses are in reclaiming rubber, dissolving casein in plastics production, refining vegetable oils, processing textiles, as an eluant in ion chromatography, etching and electroplating, and as a laboratory reagent. Sodium hydroxide also is used as a strong base in many organic synthesis and base-catalyzed reactions.
調製方法
Sodium hydroxide is manufactured by electrolysis of brine using
inert electrodes. Chlorine is evolved as a gas at the anode and
hydrogen is evolved as a gas at the cathode. The removal of chloride
and hydrogen ions leaves sodium and hydroxide ions in solution.
The solution is dried to produce the solid sodium hydroxide.
A second method uses the Kellner–Solvay cell. Saturated sodium
chloride solution is electrolyzed between a carbon anode and a
flowing mercury cathode. In this case the sodium is produced at the
cathode rather than the hydrogen because of the readiness of
sodium to dissolve in the mercury. The sodium–mercury amalgam is
then exposed to water and a sodium hydroxide solution is
produced.
定義
The most important commercial
caustic.
反応性
Sodium hydroxide is strongly alkaline and can react with acids to form salts and water.
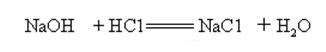
Sodium hydroxide reacts with acidic oxides to form salt and water, so sodium hydroxide can be used to absorb acid gases in the laboratory or industrially.
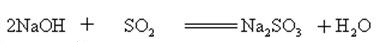
Sodium hydroxide can react with aqueous solutions of many metal salts to form sodium salts and metal hydroxides
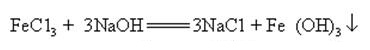
When sodium hydroxide and ammonia salt are heated together, it can release ammonia
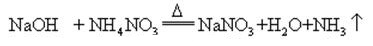
Sodium hydroxide is highly corrosive, so that the glass bottles storing sodium hydroxide solutions must be rubber stoppers, and glass stoppers should not be used to prevent a chemical reaction from opening. Sodium hydroxide is an important industrial raw material, and can be produced by electrolysis of saline solution industrially
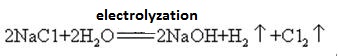
一般的な説明
A white solid. Corrosive to metals and tissue. Used in chemical manufacturing, petroleum refining, cleaning compounds, drain cleaners.
空気と水の反応
Soluble in water. Dissolution can liberate enough heat to cause steaming and spattering and ignite adjacent combustible material [Haz. Chem. Data 1966].
危険性
Corrosive to tissue in presence of mois-
ture, strong irritant to tissue (eyes, skin, mucous
membranes, and upper respiratory tract), poison by
ingestion.
健康ハザード
Sodium hydroxide is a highly corrosive substancethat causes damage to human tissues.Its action on the skin is somewhat differentfrom acid burns. There is no immediate pain,but it penetrates the skin. It does not coagulateprotein to prevent its further penetration,and thus the caustic burn can become severeand slow healing. Spilling of its concentratedsolutions into the eyes can result in severeirritation or permanent injury.
It is toxic by ingestion as well as inhalationof its dust. Although the oral toxicity ofa 5–10% solution of caustic soda was foundto be low in test animals, high dosages atgreater concentrations can cause vomiting,prostration, and collapse. The oral lethal dosein rabbits is 500 mg/kg (NIOSH 1986).
Sodium hydroxide dusts or aerosols areirritating to the eyes, nose, and throat. Prolongedexposure to high concentrations in airmay produce ulceration of the nasal passage.
火災危険
Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Some are oxidizers and may ignite combustibles (wood, paper, oil, clothing, etc.). Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated.
燃焼性と爆発性
Sodium hydroxide and potassium hydroxide are not flammable as solids or aqueous
solutions.
化学性质
强碱性物质,可以与酸性气体反应
応用例(製薬)
Sodium hydroxide is widely used in pharmaceutical formulations to
adjust the pH of solutions. It can also be used to react with weak
acids to form salts.
工業用途
Caustic soda (NaOH) is regarded as the strongest alkaline pH regulator. Caustic soda
is a very active substance and is highly corrosive. The bulk of caustic soda is manufactured
by electrolysis of saturated brines (NaCl). Caustic soda has a very strong pHregulating
capability (i.e. from pH 7 to pH 14) at a relatively low dosage compared to
other alkaline substances. Commercially, caustic soda is available in anhydrous form,
but in most mining applications the caustic soda is supplied as a 50% solution.
In the mineral processing industry, sodium hydroxide is mostly used for alkalinity control
during the processing of non-metallic minerals. In base metal flotation, the use of
sodium hydroxide is rare.
安全性プロファイル
Poison by intraperitoneal route. Moderately toxic by ingestion. Mutation data reported. A corrosive irritant to skin, eyes, and mucous membranes. When heated to decomposition it emits toxic fumes of NanO.
安全性
Sodium hydroxide is widely used in the pharmaceutical and food
industries and is generally regarded as a nontoxic material at low
concentrations. At high concentrations it is a corrosive irritant to
the skin, eyes, and mucous membranes.
LD50 (mouse, IP): 0.04 g/kg
LD50 (rabbit, oral): 0.5 g/kg
職業ばく露
NaOH is utilized to neutralize acids and make sodium salts in petroleum refining, viscose rayon; cellophane, plastic production; and in the reclamation of solutions of their salts. It is used in the manufacture of mercerized cotton, paper, explosives, and dyestuffs in metal cleaning; electrolytic extraction of zinc; tin plating; oxide coating; laundering, bleaching, dishwashing; and it is used in the chemical industries.
貯蔵
Sodium hydroxide should be stored in an airtight nonmetallic
container in a cool, dry place. When exposed to air, sodium
hydroxide rapidly absorbs moisture and liquefies, but subsequently
becomes solid again owing to absorption of carbon dioxide and
formation of sodium carbonate.
輸送方法
UN1823 NaOH, solid, Hazard class: 8; Labels: 8-Corrosive material. UN1824 NaOH, solution, Hazard class: 8; Labels: 8-Corrosive material
合成方法
通常通过电解食盐水得到
不和合性
Sodium hydroxide is a strong base and is incompatible with any
compound that readily undergoes hydrolysis or oxidation. It will
react with acids, esters, and ethers, especially in aqueous solution.
廃棄物の処理
Discharge into tank containing water, neutralize, then flush to sewer with water.
規制状況(Regulatory Status)
GRAS listed. Accepted for use as a food additive in Europe.
Included in the FDA Inactive Ingredients Database (dental
preparations; injections; inhalations; nasal, ophthalmic, oral, otic,
rectal, topical, and vaginal preparations). Included in nonparenteral
and parenteral medicines licensed in the UK. Included in the
Canadian List of Acceptable Non-medicinal Ingredients.
参考文献
H. Jacobs, J. Kockelkorn, T.Tacke, Z. Anorg. Allgem. Chem., 531, 119 (1985), DOI: 10.1002/zaac.19855311217.
水酸化ナトリウム 上流と下流の製品情報
原材料
準備製品
Sodium pyroantimonate
(+)-酒石酸ナトリウム二水和物
dibenzyl biphenyl polyoxyethylene ether
additive AC1210
2-(4,6-diamino-1,3,5-triazin-2-yl)acetic acid
2', 3'-ribonucleotide
3-(アセチルアミノ)チオフェン-2-カルボン酸
2-(1-ナフトキシ)プロピオン酸
3-フルオロ-4-ヒドロキシベンズアルデヒド
emulsifier C^{8~10^} OPE-10
thiourea-formaldehyde resin
2-チオフェンカルボン酸ナトリウム
1,7-ジオキサシクロヘプタデカン-8-オン
4-メチル-2-フェニル-1,3-チアゾール-5-カルボン酸
ビス[2-クロロ-5-[(2-ヒドロキシ-1-ナフタレニル)アゾ]-4-(ソジオスルホ)安息香酸]カルシウム
rac-(R*)-2-ヒドロキシ-2-(4-ヒドロキシ-3-メトキシフェニル)酢酸
emulsifier SOPE-20
dodecyl phenyl polyoxyethylene (12) ether
5-ブロモシトシン
C^{12~18^} fatty alcohol polyoxyethylene (35) ether
Alkaline Treated Starch
castor oil poloxyethylene (30) ether
バルビタール ナトリウム
コバルト(II)
2-ヒドロキシプロパン酸ナトリウム
Sodium isoamylxanthate
りんタングステン酸ナトリウム n水和物
2-ヒドロキシ-1-ナフトエ酸
塩化ストロンチウム六水和物
C^{8~9^} alkyl phenyl polyoxyethylene (18) ether
キヌクリジン 塩酸塩
ソーダ石灰
サフラワーイエロー
すず(IV)酸ナトリウム3水和物
2,3-ジフェニルプロピオン酸
ジチオ炭酸O-(2-メチルプロピル)=S-ナトリウム
Peregal O-25
1-カルボベンゾキシ-4-ピペリジンカルボン酸
2-アミノ-4,6-ジメトキシ-1,3,5-トリアジン
1-カルボベンゾキシピペラジン