Brexpiprazole
- CAS No.
- 913611-97-9
- Chemical Name:
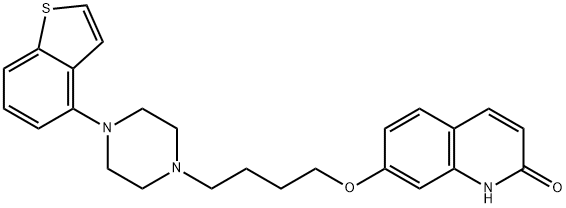
- Brexpiprazole
- Synonyms
- OPC-34712;Brexpiprazole impurity a;7-[4-(4-(Benzo[b]thien-4-yl)-piperazin-1-yl)butoxy]-1H-quinolin-2-one;7-{4-[4-(1-Benzothiophen-4-yl)piperazin-1-yl]butoxy}quinolin-2(1H)-one;7-(4-(4-(Benzo[b]thiophen-4-yl)piperazin-1-yl)butoxy)quinolin-2(1H)-one;CS-1678;ipiprazole;Epiprazole;Brexpiprazole;Brexipiprazole
- CBNumber:
- CB52681994
- Molecular Formula:
- C25H27N3O2S
- Molecular Weight:
- 433.57
- MOL File:
- 913611-97-9.mol
- MSDS File:
- SDS
- Modify Date:
- 2024/5/8 14:48:35
| Melting point | 179 - 181oC |
|---|---|
| Boiling point | 675.2±55.0 °C(Predicted) |
| Density | 1.245±0.06 g/cm3(Predicted) |
| storage temp. | Refrigerator |
| solubility | Dichloromethane (Slightly), Chloroform (Slightly), Methanol (Slightly) |
| pka | 11.22±0.70(Predicted) |
| form | Solid |
| color | White to Off-White |
| InChI | InChI=1S/C25H27N3O2S/c29-25-9-7-19-6-8-20(18-22(19)26-25)30-16-2-1-11-27-12-14-28(15-13-27)23-4-3-5-24-21(23)10-17-31-24/h3-10,17-18H,1-2,11-16H2,(H,26,29) |
| InChIKey | ZKIAIYBUSXZPLP-UHFFFAOYSA-N |
| SMILES | N1C2=C(C=CC(OCCCCN3CCN(C4=C5C=CSC5=CC=C4)CC3)=C2)C=CC1=O |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS07,GHS08,GHS09 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H372-H400-H351-H362-H360-H302-H410 | |||||||||
| Precautionary statements | P201-P202-P281-P308+P313-P405-P501-P260-P264-P270-P314-P501-P201-P260-P263-P264-P270-P308+P313-P273-P391-P501-P264-P270-P301+P312-P330-P501-P273-P391-P501 | |||||||||
| NFPA 704 |
|
Brexpiprazole Chemical Properties,Uses,Production
Description
Brexpiprazole is a novel antipsychotic drug which serves as a serotonin ® dopamine activity modulator and has demonstrated efficacy as an adjunctive treatment in patients with major depressive disorder (MDD). The drug exhibits a unique pharmacological profile, acting as a partial agonist of serotonin 5-HT1A and dopamine D2 receptors and as a full antagonist of 5-HT2A and noradrenaline α1B/2C receptors, with similar subnanomolar binding affinity. The drug, which was developed by Otsuka and Lundbeck, was approved in 2015 by the FDA for the treatment of schizophrenia and as an adjunctive treatment for depression. Brexpiprazole is widely considered to be a successor to Otsuka’s antipsychotic drug aripiprazole (trade name Abilify) whose patent expired in August 2014.
Uses
Brexpiprazole is a kind of atypical antipsychotic. It is a dopamine D2 receptor partial agonist. As a novel serotonin-dopamine activity modulator, it can be used for the treatment of schizophrenia, and for the adjunctive treatment for depression. It can also provide efficacy and tolerability over established adjunctive treatments formajor depressive disorder(MDD).Recent study has also suggested it can ameliorate PCP-induced cognitive deficits in mice via 5-HT1A receptors.
Definition
ChEBI: Brexpiprazole(913611-97-9) is a N-arylpiperazine. It is a novel D2 dopamine and serotonin 1A partial agonist, called serotonin-dopamine activity modulator (SDAM), and a potent antagonist of serotonin 2A receptors, noradrenergic alpha 1B and 2C receptors. Brexpiprazole is a drug candidate useful in treatment and prevention of mental disorders including CNS disorders.
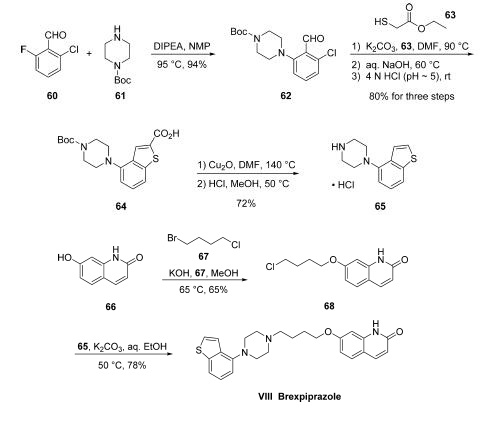
Synthesis
Commercially available fluorobenzaldehyde (60) underwent
a substitution reaction with commercial tert-butyl piperazine-1-
carboxylate (61) under basic conditions to afford the
piperazinyl benzaldehyde 62 in excellent yield. Next, the
construction of the benzothiophene was affected by initial
condensation of thioglycolic acid ethyl ester 63 with ochlorobenzaldehyde
62 under mildly basic conditions at
elevated temperatures. Treatment with aqueous base and
adjustment of pH to roughly 5 through the use of 4 N HCl
furnished the 2-carboxylic acid benzothiophene 64 in 80% yield
across the three-step operation. Next, decarboxylation through
the use of cuprous oxide using conditions slightly modified
from those originally described by Goosen followed by acidic
removal of the Boc protecting group on the terminal piperazine
nitrogen secured the key piperazinyl benzothiophene subunit
65 as the corresponding hydrochloride salt.
The hydroxyquinolone and linker component synthesis
began with alkylation of commercially available quinolone 66
with 1,4-bromochlorobutane (67) under basic conditions to
furnish chloroalkoxyquinolone 68. A subsequent alkylation with
hydrochloride salt 65 using potassium carbonate and warm
aqueous ethanol followed by recrystallizative workup resulted
in clean conversion to brexpiprazole (VIII) in 78% yield from
68.
Enzyme inhibitor
This serotonin-dopamine activity modulator, or SDAM (FW = 433.60 g/mol; CAS 913611-97-9), also known as OPC-34712, Rexulti, 7-{4-[4-(1- benzothiophen-4-yl)piperazin-1-yl]butoxy}quinolin-2(1H)-one, is an atypical antipsychotic drug that acts as a dopamine D2L (Ki = 1.1 nM) and D3 (Ki = 0.3 nM) receptor partial agonist and a partial agonist of 5-HT1A receptors (Ki = 0.12 nM). Brexpiprazole is also an antagonist of the 5-HT2A (Ki = 0.47 nM), 5-HT2B (Ki = 1.9 nM), 5-HT7 (Ki = nM), α1B-adrenergic (Ki = 0.17 nM), α2C-adrenergic (Ki = 0.59 nM), and histamine H1 receptors (Ki = 19 nM). It has negligible affinity for the mACh receptors. Rexulti is an FDA-approved add-on medication for major depressive disorder in adults. See Aripiprazole
Mode of action
Brexpiprazole's suggested mechanism of action is based on its impact on dopamine and serotonin receptors. As a serotonin-dopamine activity modulator (SDAM), it acts as a novel partial agonist for D2 dopamine and serotonin 1A receptors while effectively blocking the serotonin 2A receptors, as well as noradrenergic alpha 1B and 2C receptors.
References
https://en.wikipedia.org/wiki/Brexpiprazole
https://www.drugbank.ca/drugs/DB09128
Maeda, K, et al. "Brexpiprazole I: in vitro and in vivo characterization of a novel serotonin-dopamine activity modulator." Journal of Pharmacology & Experimental Therapeutics 350.3(2014):589-604.
Maeda, K, et al. "Brexpiprazole II: antipsychotic-like and procognitive effects of a novel serotonin-dopamine activity modulator." Journal of Pharmacology & Experimental Therapeutics 350.3(2014):605.
Yoshimi, N, et al. "Effects of brexpiprazole, a novel serotonin-dopamine activity modulator, on phencyclidine-induced cognitive deficits in mice: a role for serotonin 5-HT1A receptors. " Pharmacology Biochemistry & Behavior 124(2014):245.
Brexpiprazole Preparation Products And Raw materials
Raw materials
1of2
chevron_rightPreparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Anant Pharmaceuticals Pvt Ltd | +91-8550986868 +91-9485998001 | Haryana, India | 460 | 58 | Inquiry |
| AARTIA KEM SCIENCE | +91-8291072530 +91-8291072530 | Maharashtra, India | 70 | 58 | Inquiry |
| KARPSCHEM LABORATORIES | +91-7249203006 +91-7249203006 | Maharashtra, India | 786 | 58 | Inquiry |
| Metrochem API Private Limited | +91-4069069999 +91-4069069999 | Telangana, India | 78 | 58 | Inquiry |
| Aalidhra Pharmachem Pvt Ltd | +91-9879180894 +91-9879180894 | Gujarat, India | 38 | 58 | Inquiry |
| Alembic Pharmaceuticals Limited | +91-2652280880 +91-2652280550 | Gujarat, India | 115 | 58 | Inquiry |
| Honour Lab Limited | +919845977466 | Telangana, India | 164 | 58 | Inquiry |
| Unichem Laboratories Ltd | +91-2266888333 +91-2266888333 | Maharashtra, India | 62 | 58 | Inquiry |
| Anlon Healthcare Pvt Ltd | +91-2812562538 +91-7069690081 | Gujarat, India | 54 | 58 | Inquiry |
| Zydus Lifesciences Ltd. | +91-0265-2315243 +91-6358895661 | Gujarat, India | 89 | 58 | Inquiry |
Related articles
- Brexpiprazole: Effective and Well-Tolerated Treatment in Schizophrenia Patients
- Brexpiprazole is an effective and well-tolerated treatment option for acute schizophrenia and maintenance treatment in adults.
- Jan 9,2024
- Brexpiprazole: mechanism of action, pharmacokinetics, application and side effect
- Brexpiprazole is used for schizophrenia and MDD. It acts on multiple receptors, has long half-life and interacts with CYP enzy....
- Jul 12,2023
- Brexpiprazole-an Antipsychotic Medication
- Brexpiprazole is an antipsychotic medication. It works by changing the actions of chemicals in the brain. It is used to treat ....
- Nov 12,2019





